Abstract
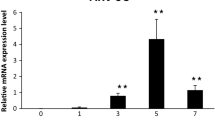
Avian Tembusu virus (ATV), an emerging virus that mainly infects laying and breeding ducks in China, has caused severe economic loss in duck industry. However, there have been no reports about host innate immune responses during ATV infection and its correlation with clinical signs or pathology. To identify the roles of these immune factors in the innate host response to ATV infection, quantitative real-time PCR (qPCR) was used to analyze the transcriptional profiles on the genes encoding two retinoic-acid-induced gene I (RIG-I)-like receptors (RLRs) and two interferons (INF-α and INF-γ) in seven tissues of an ATV-infected shelduck. After infection with ATV, both RLR genes were significantly upregulated (P < 0.05) in all seven tissues. The peak expression levels of the two RLR genes were observed at 24 hours postinfection (hpi) and were higher in non-lymphoid tissues (liver, lung, kidney, and ovary) than in lymphoid tissues (thymus, spleen and bursa). Although the transcription levels of both IFN genes were also upregulated, they showed different time-dependent expression patterns compared with those of the RLR genes. In addition, the highest mRNA expression of the two IFN genes was observed in the ovary at 6 hpi. This observation suggests that the ovary is the primary target tissue in ATV infection and explains the clinical characteristics of the primary pathological changes in the ovaries of ATV-infected ducks. Our results, for the first time, elucidate the differential and coordinated expression profiles of two RLRs and two IFNs in an ATV-infected shelduck.




Similar content being viewed by others
References
Cao Z, Zhang C, Liu Y, Liu Y, Ye W, Han J, Ma G, Zhang D, Xu F, Gao X, Tang Y, Shi S, Wan C, Zhang C, He B, Yang M, Lu X, Huang Y, Diao Y, Ma X, Zhang D (2011) Tembusu virus in ducks, China. Emerg Infect Dis 17:1873–1875
Su J, Li S, Hu X, Yu X, Wang Y, Liu P, Lu X, Zhang G, Liu D, Li X, Su W, Lu H, Mok NS, Wang P, Wang M, Tian K, Gao GF (2011) Duck egg-drop syndrome caused by BYD virus, a new Tembusu-related flavivirus. PLoS One 6:e18106
Wan C, Shi S, Cheng L, Chen H, Fu G, Zhang D, Lin F, Lin J, Huang Y (2010) A newly identified Flavivirus Virus causing abrupt egg-laying reduction in ducks. Fujian J Agric Sci 25:663–666
Yan P, Zhao Y, Zhang X, Xu D, Dai X, Teng Q, Yan L, Zhou J, Ji X, Zhang S, Liu G, Zhou Y, Kawaoka Y, Tong G, Li Z (2011) An infectious disease of ducks caused by a newly emerged Tembusu virus strain in mainland China. Virology 417:1–8
Fu G, Huang Y, Cheng L, Wan C, Shi S, Fu Q, Chen H, Lin J, Lin F (2014) Genome sequence and phylogenetic analysis of Tembusu viruses isolated from chicken. Chin J Vet Sci 34:1418–1422
Liu Y, Peng C, Fu G, Hou D, Shi S, Wan C, Cheng L, Chen H, Lin J, Lin F (2012) Detection and molecular analysis of avian Tembusu virus partial areas of China from 2010 to 2011. Chin J Anim Infect Dis 6:47–53
Tang Y, Diao Y, Yu C, Gao X, Ju X, Xue C, Liu X, Ge P, Qu J, Zhang D (2013) Characterization of a Tembusu virus isolated from naturally infected house sparrows (Passer domesticus) in Northern China. Transbound Emerg Dis 60:152–158
Liu P, Lu H, Li S, Moureau G, Deng YQ, Wang Y, Zhang L, Jiang T, de Lamballerie X, Qin CF, Gould EA, Su J, Gao GF (2012) Genomic and antigenic characterization of the newly emerging Chinese duck egg-drop syndrome flavivirus: genomic comparison with Tembusu and Sitiawan viruses. J Gen Virol 93:2158–2170
De Andrea M, Ravera R, Gioia D, Gariglio M, Landolfo S (2002) The interferon system: an overview. Eur J Paediatr Neurol 6(Suppl A):A41–A46 (discussion A55–58)
Loo YM, Gale M Jr (2011) Immune signaling by RIG-I-like receptors. Immunity 34:680–692
Matsumiya T, Stafforini DM (2010) Function and regulation of retinoic acid-inducible gene-I. Crit Rev Immunol 30:489–513
Yoo JS, Kato H, Fujita T (2014) Sensing viral invasion by RIG-I like receptors. Curr Opin Microbiol 20:131–138
Kawai T, Akira S (2009) The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol 21:317–337
Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S (2006) Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101–105
Loo YM, Fornek J, Crochet N, Bajwa G, Perwitasari O, Martinez-Sobrido L, Akira S, Gill MA, Garcia-Sastre A, Katze MG, Gale M Jr (2008) Distinct RIG-I and MDA5 signaling by RNA viruses in innate immunity. J Virol 82:335–345
Myong S, Cui S, Cornish PV, Kirchhofer A, Gack MU, Jung JU, Hopfner KP, Ha T (2009) Cytosolic viral sensor RIG-I is a 5’-triphosphate-dependent translocase on double-stranded RNA. Science 323:1070–1074
Weber M, Weber F (2014) RIG-I-like receptors and negative-strand RNA viruses: RLRly bird catches some worms. Cytokine Growth Factor Rev 25:621–628
Fredericksen BL, Gale M Jr (2006) West Nile virus evades activation of interferon regulatory factor 3 through RIG-I-dependent and -independent pathways without antagonizing host defense signaling. J Virol 80:2913–2923
Barber MR, Aldridge JR Jr, Webster RG, Magor KE (2010) Association of RIG-I with innate immunity of ducks to influenza. Proc Natl Acad Sci USA 107:5913–5918
Wilkins C, Gale M Jr (2010) Recognition of viruses by cytoplasmic sensors. Curr Opin Immunol 22:41–47
Chen L, Fu G, Huang Y, Qi B, Fu Q, Shi S, Cheng L, Wan C, Chen H, Chen C (2013) Distribution and shedding of duck Tembusu virus in experimentally infected sheldrake ducks. Chin J Anim Infect Dis 21:20–24
Reaiche GY (2008) Characterisation of the events involved in the resolution of acute duck Hepatitis B virus infection. Dissertation, University of Adelaide
Song C, Yu S, Duan Y, Hu Y, Qiu X, Tan L, Sun Y, Wang M, Cheng A, Ding C (2014) Effect of age on the pathogenesis of DHV-1 in Pekin ducks and on the innate immune responses of ducks to infection. Arch Virol 159:905–914
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2[-Delta Delta C(T)] Method. Methods 25:402–408
Chen Lei, Guanghua Fu, Qi Baomin, Shi Shaohua, Wan Chunhe, Cheng Longfei, Chen Hongmei, Qiuling Fu, Huang Yu (2013) The pathologic changes of Sheldrake duck infected with Tembusu virus. Fujian J Agric Sci 28(5):423–426
Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, Endres S, Hartmann G (2006) 5’-Triphosphate RNA is the ligand for RIG-I. Science 314:994–997
Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, Weber F, Reis e Sousa C (2006) RIG-I-mediated antiviral responses to single-stranded RNA bearing 5’-phosphates. Science 314:997–1001
Sen A, Pruijssers AJ, Dermody TS, Garcia-Sastre A, Greenberg HB (2011) The early interferon response to rotavirus is regulated by PKR and depends on MAVS/IPS-1, RIG-I, MDA-5, and IRF3. J Virol 85:3717–3732
Baum A, Sachidanandam R, Garcia-Sastre A (2010) Preference of RIG-I for short viral RNA molecules in infected cells revealed by next-generation sequencing. Proc Natl Acad Sci USA 107:16303–16308
Schoenborn JR, Wilson CB (2007) Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol 96:41–101
Miossec P, Korn T, Kuchroo VK (2009) Interleukin-17 and type 17 helper T cells. N Engl J Med 361:888–898
Navratil V, de Chassey B, Meyniel L, Pradezynski F, Andre P, Rabourdin-Combe C, Lotteau V (2010) System-level comparison of protein-protein interactions between viruses and the human type I interferon system network. J Proteome Res 9:3527–3536
Lin RJ, Liao CL, Lin E, Lin YL (2004) Blocking of the alpha interferon-induced Jak-Stat signaling pathway by Japanese encephalitis virus infection. J Virol 78:9285–9294
Acknowledgments
The authors gratefully acknowledge Dr. Oliver He (University of Michigan) for helping us edit the manuscript, and Edison for his statistical guidance on this work. This work was supported by the National Natural Science Foundation of China (No. 31201936), the Science Foundation of Two Sides of Strait (No. U1305212), the Earmarked Fund for Modern Agro-Industry Technology Research System (CARS-43) from the Ministry of Agriculture of P. R. China, and the Program of Science and Technology Young Talents (YC2015-13) from Fujian Academy of Agricultural Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
G. Fu and C. Chen contributed equally to this study.
Rights and permissions
About this article
Cite this article
Fu, G., Chen, C., Huang, Y. et al. Comparative analysis of transcriptional profiles of retinoic-acid-induced gene I-like receptors and interferons in seven tissues from ducks infected with avian Tembusu virus. Arch Virol 161, 11–18 (2016). https://doi.org/10.1007/s00705-015-2621-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-015-2621-x