Abstract
Background
Palonosetron is the second-generation 5-hydroxytryptamine 3 receptor antagonist (5-HT3RA) that has shown better efficacy than the first-generation 5-HT3RA for prevention of chemotherapy-induced nausea and vomiting (CINV) in patients receiving moderately emetogenic chemotherapy (MEC). Granisetron transdermal delivery system (GTDS), a novel transdermal formulation, was developed to deliver granisetron continuously over 7 days. This study compared the efficacy and tolerability of the GTDS to palonosetron for the control of CINV following MEC.
Material and method
A total of 196 patients were randomized to GP or PG group. In this multicenter, randomized, open-label, cross-over, active-controlled, Phase IV study, GP group was assigned to receive transdermal granisetron (one GTDS patch, 7 days) in the first chemotherapy cycle, palonosetron (iv 0.25 mg/day, 1 days) in the second chemotherapy cycle before receiving MEC, and PG group was assigned to receive palonosetron in the first cycle and GTDS in the second cycle. Primary endpoint was the percentage of chemotherapy cycles achieving complete response (CR; defined as no emetic episodes and no rescue medication use) during the acute phase (0–24 h in post-chemotherapy; non-inferiority comparison with palonosetron).
Results
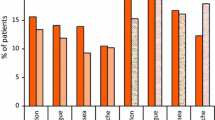
Total 333 cycles (165 in GTDS and 168 in palonosetron) were included in the per protocol analysis. The GTDS cycles showed non-inferiority to palonosetron cycles during the acute phase: CR was achieved by 124 (75.2 %) patients in the GTDS cycles and 134 (79.8 %) patients in the palonosetron cycles (treatment difference, −4.6 %; 95 % confidence interval, −13.6–4.4). There was no significant difference in CR rate during acute phase after the end of the first and second chemotherapy cycle between GP and PG group (p = 0.405, p = 0.074). Patients’ satisfaction, assessed using Functional Living Index-Emesis (FLI-E), GTDS cycle were higher than those of palonosetron cycle in GP group (FLI-E score; median 1549.5 in GTDS cycle, median 1670.0 in palonosetron cycle). Both treatments were well tolerated and safe.
Conclusion
Transdermal granisetron is a good alternative therapeutic option to palonosetron for preventing CINV after MEC.



Similar content being viewed by others
References
Griffin AM, Butow PN, Coates AS, et al (1996) On the receiving end. V: patient perceptions of the side effects of cancer chemotherapy in 1993. Ann Oncol 7:189–195
Almeida EP, Gutierrez MG, Adami NP (2004) Monitoring and evaluation of side effects of chemotherapy in patients with colon cancer. Rev Lat Am Enfermagem 12:760–766
Andrews PL, Sanger GJ (2002) Abdominal vagal afferent neurones: an important target for the treatment of gastrointestinal dysfunction. Curr Opin Pharmacol 2:650–656
Fukui H, Yamamoto M, Sato S (1992) Vagal afferent fibers and peripheral 5-HT3 receptors mediate cisplatin-induced emesis in dogs. Jpn J Pharmacol 59:221–226
Balfour JA, Goa KL (1997) Dolasetron. A review of its pharmacology and therapeutic potential in the management of nausea and vomiting induced by chemotherapy, radiotherapy or surgery. Drugs 54:273–298
Hasler WL (1999) Serotonin receptor physiology: relation to emesis. Dig Dis Sci 44(8 suppl):108S–113S
Hesketh PJ (2000) Comparative review of 5-HT3 receptor antagonists in the treatment of acute chemotherapy-induced nausea and vomiting. Cancer Investig 18:163–173
Forni C, Ferrari S, Loro L, et al (2000) Granisetron, tropisetron, and ondansetron in the prevention of acute emesis induced by a combination of cisplatin–adriamycin and by high-dose ifosfamide delivered in multi-day continuous infusions. Support Care Cancer 8:131–133
Gralla RJ, Osoba D, Kris MG, et al (1999) Recommendations for the use of antiemetics: evidence-based, clinical practice guidelines. J Clin Oncol 17:2971–2994
Koeller JM, Aapro MS, Gralla RJ, et al (2002) Antiemetic guidelines: creating a more practical approach. Support Care Cancer 10:519–522
Kaizer L, Warr D, Hoskins P, et al (1994) Effect of schedule and maintenance on the antiemetic efficacy of ondansetron combined with dexamethasone in acute and delayed nausea and emesis in patients receiving moderately emetogenic chemotherapy: a phase III trial by the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 12:1050–1057
Howell J, Smeets J, Drenth HJ, Gill D (2009) Pharmacokinetics of a granisetron transdermal system for the treatment of chemotherapy-induced nausea and vomiting. J Oncol Pharm Pract 15:223–231
Boccia RV, Gordan LN, Clark G, et al (2011) Sancuso Study Group. Efficacy and tolerability of transdermal granisetron for the control of chemotherapy-induced nausea and vomiting associated with moderately and highly emetogenic multi-day chemotherapy: a randomized, double-blind, phase III study. Support Care Cancer 19(10):1609–1617
Aapro MS, Grunberg SM, Manikhas GM, et al (2006) A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol 17:1441–1449
Eisenberg P, Figueroa-Vadillo J, Zamora R, et al (2003) Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetron. Cancer 98:2473–2482
Eisenberg P, MacKintosh FR, Ritch P, Cornett PA, Macciocchi A (2004) Efficacy, safety and pharmacokinetics of palonosetron in patients receiving highly emetogenic cisplatin-based chemotherapy: a dose ranging clinical study. Ann Oncol 15:330–337
Gralla R, Lichinitser M, Van Der Vegt S, et al (2003) Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol 14:1570–1577
Botrel TE, Clark OA, Clark L, et al (2011) Efficacy of palonosetron (PAL) compared to other serotonin inhibitors (5-HT3R) in preventing chemotherapy-induced nausea and vomiting (CINV) in patients receiving moderately or highly emetogenic (MoHE) treatment: systematic review and meta-analysis. Support Care Cancer 19:823–832
Kraut L, Fauser AA (2001) Anti-emetics for cancer chemotherapy induced emesis: potential of alternative delivery systems. Drugs 61:1553–1562
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Seol, Y.M., Kim, H.J., Choi, Y.J. et al. Transdermal granisetron versus palonosetron for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: a multicenter, randomized, open-label, cross-over, active-controlled, and phase IV study. Support Care Cancer 24, 945–952 (2016). https://doi.org/10.1007/s00520-015-2865-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-015-2865-8