Abstract
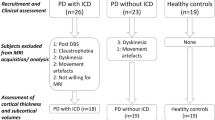
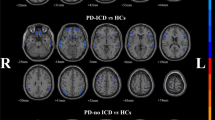
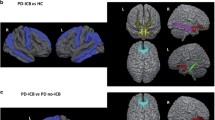
Impulse control disorders (ICDs) occur in a subset of patients with Parkinson’s disease (PD) who are receiving dopamine replacement therapy. In this study, we aimed to investigate structural abnormalities within the mesocortical and limbic cortices and subcortical structures in PD patients with ICDs. We studied 18 PD patients with ICDs, 18 PD patients without ICDs and a group of 24 age and sex-matched healthy controls. Cortical thickness (CTh) and subcortical nuclei volume analyses were carried out using the automated surface-based analysis package FreeSurfer (version 5.3.0). We found significant differences in MRI measures between the three groups. There was volume loss in the nucleus accumbens of both PD patients with ICDs and without ICDs compared to the control group. In addition, PD patients with ICDs showed significant atrophy in caudate, hippocampus and amygdala compared to the group of healthy controls. PD patients with ICDs had significant increased cortical thickness in rostral anterior cingulate cortex and frontal pole compared to PD patients without ICDs. Cortical thickness in rostral anterior cingulate and frontal pole was increased in PD patients with ICDs compared to the control group, but the differences failed to reach corrected levels of statistical significance. PD patients with ICDs showed increased cortical thickness in medial prefrontal regions. We speculate that these findings reflect either a pre-existing neural trait vulnerability to impulsivity or the expression of a maladaptive synaptic plasticity under non-physiological dopaminergic stimulation.

Similar content being viewed by others
References
Wu K, Politis M, Piccini P (2009) Parkinson disease and impulse control disorders: a review of clinical features, pathophysiology and management. Postgrad Med J 85:590–596
Weintraub D, Koester J, Potenza MN et al (2010) Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol 67:589–595
Leeman RF, Billingsley BE, Potenza MN (2012) Impulse control disorders in Parkinson’s disease: background and update on prevention and management. Neurodegener Dis Manag 2:389–400
O’Sullivan SS, Wu K, Politis M et al (2011) Cue-induced striatal dopamine release in Parkinson’s disease-associated impulsive–compulsive behaviours. Brain 134:969–978
Steeves TD, Miyasaki J, Zurowski M et al (2009) Increased striatal dopamine release in Parkinsonian patients with pathological gambling: a [11C] raclopride PET study. Brain 132:1376–1385
Cilia R, Cho SS, van Eimeren T et al (2011) Pathological gambling in patients with Parkinson’s disease is associated with fronto-striatal disconnection: a path modeling analysis. Mov Disord 26:225–233
vanEimeren T, Pellecchia G, Cilia R et al (2010) Drug-induced deactivation of inhibitory networks predicts pathological gambling in PD. Neurology 75:1711–1716
Voon V, Pessiglione M, Brezing C et al (2010) Mechanisms underlying dopamine-mediated reward bias in compulsive behaviors. Neuron 65:135–142
Politis M, Loane C, Wu K et al (2013) Neural response to visual sexual cues in dopamine treatment-linked hypersexuality in Parkinson’s disease. Brain 136:400–411
Caprioli D, Sawiak SJ, Merlo E et al (2014) Gamma aminobutyric acidergic and neuronal structural markers in the nucleus accumbens core underlie trait-like impulsive behavior. Biol Psychiatry 75:115–123
Wheeler AL, Lerch JP, Chakravarty MM et al (2013) Adolescent cocaine exposure causes enduring macroscale changes in mouse brain structure. J Neurosci 33:1797–1803a
Cerasa A, Salsone M, Nigro S et al (2014) Cortical volume and folding abnormalities in Parkinson’s disease patients with pathological gambling. Parkinsonism Relat Disord. pii: S1353-8020(14)00323-X [Epub ahead of print]
Biundo R, Weis L, Facchini S et al (2015) Patterns of cortical thickness associated with impulse control disorders in Parkinson’s disease. Mov Disord. doi:10.1002/mds.26154 [Epub ahead of print]
Gelb DJ, Oliver E, Gilman S (1999) Diagnostic criteria for Parkinson disease. Arch Neurol 56:33–39
Weintraub D, Hoops S, Shea JA et al (2009) Validation of the questionnaire for impulsive–compulsive disorders in Parkinson’s disease. Mov Disord 24:1461–1467
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders, 4th edn. American Psychiatric Association, Washington, DC
Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease (2013) The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov Disord 18:738–750
Goetz CG, Poewe W, Rascol O et al (2004) Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord 19:1020–1028
Folstein MF, Folstein SE, McHugh PR (1975) “Mini Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiat Res 12:189–198
Politis M, Wu K, Loane C et al (2010) Staging of serotonergic dysfunction in Parkinson’s disease: an in vivo 11C-DASB PET study. Neurobiol Dis 40:216–221
Reuter M, Tisdall MD, Qureshi A, Buckner RL, van der Kouwe AJ, Fischl B (2015) Head motion during MRI acquisition reduces gray matter volume and thickness estimates. Neuroimage 107:107–115
Fischl B, Salat DH, van der Kouwe AJ et al (2004) Sequence-independent segmentation of magnetic resonance images. Neuroimage 23(Suppl 1):S69–S84
Fischl B, Salat DH, Busa E et al (2002) Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355
Cardinale F, Chinnici G, Bramerio M et al (2014) Validation of FreeSurfer-estimated brain cortical thickness: comparison with histologic measurements. Neuroinformatics 12:535–542
Mulder ER, de Jong RA, Knol DL (2014) Hippocampal volume change measurement: quantitative assessment of the reproducibility of expert manual outlining and the automated methods FreeSurfer and FIRST. Neuroimage 92:169–181
Malone IB, Leung KK, Clegg S et al (2015) Accurate automatic estimation of total intracranial volume: a nuisance variable with less nuisance. Neuroimage 104:366–372
Robinson TE, Berridge KC (2003) Addiction. Annu Rev Psychol 54:25–53
Haber SN (2014) The place of dopamine in the cortico-basal ganglia circuit. Neuroscience 282:248–257
Berridge KC (2007) The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology 191:391–431
Schultz W (2011) Potential vulnerabilities of neuronal reward, risk, and decision mechanisms to addictive drugs. Neuron 69:603–617
Hill SY, De Bellis MD, Keshavan MS et al (2001) Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biol Psychiatry 49:894–905
Muhlert N, Lawrence AD (2015) Brain structure correlates of emotion-based rash impulsivity. Neuroimage 115:138–146
Price JL (2005) Free will versus survival: brain systems that underlie intrinsic constraints on behavior. Comp Neurol 493:132–139
Kringelbach ML (2005) The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci 6:691–702
Rushworth MF, Kolling N, Sallet J, Mars RB (2012) Valuation and decision-making in frontal cortex: one or many serial or parallel systems? Curr Opin Neurobiol 22:946–955
Cho SS, Pellecchia G, Aminian K et al (2013) Morphometric correlation of impulsivity in medial prefrontal cortex. Brain Topogr 26:479–487
Politis M, Wu K, Loane C et al (2014) Serotonergic mechanisms responsible for levodopa-induced dyskinesias in Parkinson’s disease patients. J Clin Invest 124:1340–1349
Voon V, Fernagut PO, Wickens J et al (2009) Chronic dopaminergic stimulation in Parkinson’s disease: from dyskinesias to impulse control disorders. Lancet Neurol 8:1140–1149
Cerasa A, Messina D, Pugliese P et al (2011) Increased prefrontal volume in PD with levodopa-induced dyskinesias: a voxel-based morphometry study. Mov Disord 26:807–812
Cerasa A, Morelli M, Augimeri A et al (2013) Prefrontal thickening in PD with levodopa-induced dyskinesias: new evidence from cortical thickness measurement. Parkinsonism Relat Disord 19:123–125
Morgante F, Espay AJ, Gunraj C, Lang AE, Chen R (2006) Motor cortex plasticity in Parkinson’s disease and levodopa-induced dyskinesias. Brain 129:1059–1069
Cerasa A, Fasano A, Morgante F et al (2014) Maladaptive plasticity in levodopa-induced dyskinesias and tardive dyskinesias: old and new insights on the effects of dopamine receptor pharmacology. Front Neurol 9(5):49
Vernon AC, Modo M (2012) Do levodopa treatments modify the morphology of the parkinsonian brain? Mov Disord 27:166–167
Finlay CJ, Duty S, Vernon AC (2014) Brain morphometry and the neurobiology of levodopa-induced dyskinesias: current knowledge and future potential for translational pre-clinical neuroimaging studies. Front Neurol 5:95
Salgado-Pineda P, Delaveau P, Falcon C, Blin O (2006) Brain T1 intensity changes after levodopa administration in healthy subjects: a voxel-based morphometry study. Br J Clin Pharmacol 62:546–551
Kolb B, Gibb R (2015) Plasticity in the prefrontal cortex of adult rats. Front Cell Neurosci 9:15
Acknowledgments
We thank all participants and their families. Marios Politis research is supported by Parkinson’s UK, Lily and Edmond J. Safra Foundation, Michael J. Fox Foundation (MJFF), and NIHR BRC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical standard
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review boards and the local research ethics committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Pellicano, C., Niccolini, F., Wu, K. et al. Morphometric changes in the reward system of Parkinson’s disease patients with impulse control disorders. J Neurol 262, 2653–2661 (2015). https://doi.org/10.1007/s00415-015-7892-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-015-7892-3