Abstract
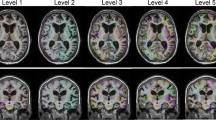
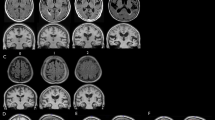
Evidence suggests that normal pressure hydrocephalus (NPH) is underdiagnosed in day to day radiologic practice, and differentiating NPH from cerebral atrophy due to other neurodegenerative diseases and normal aging remains a challenge. To better characterize NPH, we test the hypothesis that a prediction model based on automated MRI brain tissue segmentation can help differentiate shunt-responsive NPH patients from cerebral atrophy due to Alzheimer disease (AD) and normal aging. Brain segmentation into gray and white matter (GM, WM), and intracranial cerebrospinal fluid was derived from pre-shunt T1-weighted MRI of 15 shunt-responsive NPH patients (9 men, 72.6 ± 8.0 years-old), 17 AD patients (10 men, 72.1 ± 11.0 years-old) chosen as a representative of cerebral atrophy in this age group; and 18 matched healthy elderly controls (HC, 7 men, 69.7 ± 7.0 years old). A multinomial prediction model was generated based on brain tissue volume distributions. GM decrease of 33 % relative to HC characterized AD (P < 0.005). High preoperative ventricular and near normal GM volumes characterized NPH. A multinomial regression model based on gender, GM and ventricular volume had 96.3 % accuracy differentiating NPH from AD and HC. In conclusion, automated MRI brain tissue segmentation differentiates shunt-responsive NPH with high accuracy from atrophy due to AD and normal aging. This method may improve diagnosis of NPH and improve our ability to distinguish normal from pathologic aging.




Similar content being viewed by others
Abbreviations
- NPH:
-
Normal pressure hydrocephalus
- AD:
-
Alzheimer disease
- HC:
-
Healthy control
- CSF:
-
Cerebrospinal fluid
- GM:
-
Gray matter
- WM:
-
White matter
References
Adams RD et al (1965) Symptomatic occult hydrocephalus with “Normal” cerebrospinal-fluid pressure. A treatable syndrome. N Engl J Med 273:117–126
Finney GR (2009) Normal pressure hydrocephalus. Int Rev Neurobiol 84:263–281
Aygok G, Marmarou A, Young HF (2005) Three-year outcome of shunted idiopathic NPH patients. Acta Neurochir Suppl 95:241–245
Malm J et al (2000) Three-year survival and functional outcome of patients with idiopathic adult hydrocephalus syndrome. Neurology 55(4):576–578
Meier U, Miethke C (2003) Predictors of outcome in patients with normal-pressure hydrocephalus. J Clin Neurosci 10(4):453–459
Kahlon B, Sjunnesson J, Rehncrona S (2007) Long-term outcome in patients with suspected normal pressure hydrocephalus. Neurosurgery 60(2):327–332 (discussion 332)
Tisell M et al (2006) Long-term outcome in 109 adult patients operated on for hydrocephalus. Br J Neurosurg 20(4):214–221
Hebb AO, Cusimano MD (2001) Idiopathic normal pressure hydrocephalus: a systematic review of diagnosis and outcome. Neurosurgery 49(5):1166–1184 (discussion 1184–1186)
Leinonen V et al (2012) Post-mortem findings in 10 patients with presumed normal-pressure hydrocephalus and review of the literature. Neuropathol Appl Neurobiol 38(1):72–86
Ishikawa M et al (2008) Guidelines for management of idiopathic normal pressure hydrocephalus. Neurol Med Chir (Tokyo) 48(Suppl):S1–S23
George AE et al (1995) The differential diagnosis of Alzheimer’s disease. Cerebral atrophy versus normal pressure hydrocephalus. Neuroimaging Clin N Am 5(1):19–31
Holodny AI et al (1998) Focal dilation and paradoxical collapse of cortical fissures and sulci in patients with normal-pressure hydrocephalus. J Neurosurg 89(5):742–747
Lee WJ et al (2010) Brain MRI as a predictor of CSF tap test response in patients with idiopathic normal pressure hydrocephalus. J Neurol 257(10):1675–1681
Tarnaris A, Kitchen ND, Watkins LD (2009) Noninvasive biomarkers in normal pressure hydrocephalus: evidence for the role of neuroimaging. J Neurosurg 110(5):837–851
Toma AK et al (2011) Evans’ index revisited: the need for an alternative in normal pressure hydrocephalus. Neurosurgery 68(4):939–944
Rusinek H et al (1991) Alzheimer disease: measuring loss of cerebral gray matter with MR imaging. Radiology 178(1):109–114
George AE et al (1981) Parenchymal CT correlates of senile dementia (Alzheimer disease): loss of gray-white matter discriminability. AJNR Am J Neuroradiol 2(3):205–213
Nelson AJ (1976) Analysis of movement through utilisation of clinical instrumentation. Physiotherapy 62(4):123–124
Nelson AJ et al (2002) The validity of the GaitRite and the Functional Ambulation Performance scoring system in the analysis of Parkinson gait. NeuroRehabilitation 17(3):255–262
Hauser SL et al (1983) Immunosuppression and plasmapheresis in chronic progressive multiple sclerosis. Design of a clinical trial. Arch Neurol 40(11):687–690
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12(3):189–198
Reisberg B et al (1982) The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry 139(9):1136–1139
Fisher CM (1982) Hydrocephalus as a cause of disturbances of gait in the elderly. Neurology 32(12):1358–1363
Graff-Radford NR, Godersky JC (1986) Normal-pressure hydrocephalus. Onset of gait abnormality before dementia predicts good surgical outcome. Arch Neurol 43(9):940–942
Golomb J et al (2000) Alzheimer’s disease comorbidity in normal pressure hydrocephalus: prevalence and shunt response. J Neurol Neurosurg Psychiatry 68(6):778–781
Ashburner J, Friston KJ (2005) Unified segmentation. Neuroimage 26(3):839–851
Mikheev A et al (2008) Fully automatic segmentation of the brain from T1-weighted MRI using Bridge Burner algorithm. J Magn Reson Imaging 27(6):1235–1241
AA (2002) Categorical Data Analysis, 2nd edn. John Wiley & Sons, New York
Tanabe JL et al (1997) Tissue segmentation of the brain in Alzheimer disease. AJNR Am J Neuroradiol 18(1):115–123
Karas GB et al (2003) A comprehensive study of gray matter loss in patients with Alzheimer’s disease using optimized voxel-based morphometry. Neuroimage 18(4):895–907
Balthazar ML et al (2009) Differences in grey and white matter atrophy in amnestic mild cognitive impairment and mild Alzheimer’s disease. Eur J Neurol 16(4):468–474
Ishii K et al (2008) Voxel-based analysis of gray matter and CSF space in idiopathic normal pressure hydrocephalus. Dement Geriatr Cogn Disord 25(4):329–335
Moore DW et al (2012) A pilot study of quantitative MRI measurements of ventricular volume and cortical atrophy for the differential diagnosis of normal pressure hydrocephalus. Neurol Res Int 2012:718150
Kang K et al (2013) Idiopathic normal-pressure hydrocephalus, cortical thinning, and the cerebrospinal fluid tap test. J Neurol Sci 334(1–2):55–62
Bradley WG Jr et al (1996) Normal-pressure hydrocephalus: evaluation with cerebrospinal fluid flow measurements at MR imaging. Radiology 198(2):523–529
Bradley WG Jr (2001) Diagnostic tools in hydrocephalus. Neurosurg Clin N Am 12(4): 661–684, viii
Walchenbach R et al (2002) The value of temporary external lumbar CSF drainage in predicting the outcome of shunting on normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry 72(4):503–506
Marmarou A et al (2005) The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus. Neurosurgery 57(3 Suppl):S17–S28 (discussion ii–v)
Wikkelso C et al (2013) The European iNPH Multicentre Study on the predictive values of resistance to CSF outflow and the CSF Tap Test in patients with idiopathic normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry 84(5):562–568
Bradley WG (2000) Normal pressure hydrocephalus: new concepts on etiology and diagnosis. AJNR Am J Neuroradiol 21(9):1586–1590
Holodny AI et al (1998) MR differential diagnosis of normal-pressure hydrocephalus and Alzheimer disease: significance of perihippocampal fissures. AJNR Am J Neuroradiol 19(5):813–819
Hattori T et al (2012) White matter alteration in idiopathic normal pressure hydrocephalus: tract-based spatial statistics study. AJNR Am J Neuroradiol 33(1):97–103
Osuka S et al (2010) Diffusion tensor imaging in patients with adult chronic idiopathic hydrocephalus. Neurosurgery 67(5):E1474
Hattingen E et al (2010) Diffusion tensor imaging in patients with adult chronic idiopathic hydrocephalus. Neurosurgery 66(5):917–924
Kim MJ et al (2011) Differential diagnosis of idiopathic normal pressure hydrocephalus from other dementias using diffusion tensor imaging. AJNR Am J Neuroradiol 32(8):1496–1503
Acknowledgments
This research was supported by the National Institutes of Health Grants AG012101, AG027852, AG08051, EB01015 and NS050520. We wish to acknowledge the assistance of Dr. Joseph A. Helpern in providing data obtained from his grants (AG 027852 and The Litwin Foundation) for analysis, and Patricia Tolete for her assistance in reviewing medical records.
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflicts of interest.
Ethical Standards
This study has been performed in accordance with the standards laid down in the Declaration of Helsinki.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Serulle, Y., Rusinek, H., Kirov, I.I. et al. Differentiating shunt-responsive normal pressure hydrocephalus from Alzheimer disease and normal aging: pilot study using automated MRI brain tissue segmentation. J Neurol 261, 1994–2002 (2014). https://doi.org/10.1007/s00415-014-7454-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-014-7454-0