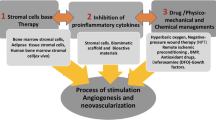
Abstract
Introduction
The creation of axially vascularized bone substitutes (AVBS) has been successfully demonstrated in several animal models. One prototypical indication is bone replacement in patients with previously irradiated defect sites, such as in the mandibular region. The downside of current clinical practice, when free fibular or scapular grafts are used, is the creation of significant donor site morbidity.
Methods
Based on our previous experiments, we extended the creation of an arterio-venous loop to generate vascularized bone substitutes to a new defect model in the goat mandibula. In this report, we review the literature regarding different models for axially vascularized bone substitutes and present a novel model demonstrating the feasibility of combining this model with synthetic porous scaffold materials and biological tissue adhesives to grow cells and tissue.
Results
We were able to show the principal possibility to generate axially vascularized bony substitutes in vivo in goat mandibular defects harnessing the regenerative capacity of the living organism and completely avoiding donor site morbidity.
Conclusion
From our findings, we conclude that this novel model may well offer new perspectives for orthopedic and traumatic bone defects that might benefit from the reduction of donor site morbidity.








Similar content being viewed by others
References
Giannoudis PV, Dinopoulos H, Tsiridis E (2005) Bone substitutes: an update. Injury 36:20
Rogers SN, Lakshmiah SR, Narayan B et al (2003) A comparison of the long term morbidity following deep circumflex iliac and fibula free flaps for reconstruction following head and neck cancer. Plast Reconstr Surg 112:1517
Hartman EH, Spauwen PH, Jansen JA (2002) Donor-site complications in vascularized bone flap surgery. J Invest Surg 15:185
Hodde J (2002) Naturally occurring scaffolds for soft tissue repair and regeneration. Tissue Eng 8:295
Bleiziffer O, Hammon M, Naschberger E, Lipnik K, Arkudas A, Rath S, Pryymachuk G, Beier JP, Stürzl M, Horch RE, Kneser U (2011) Endothelial progenitor cells are integrated in newly formed capillaries and alter adjacent fibrovascular tissue after subcutaneous implantation in a fibrin matrix. J Cell Mol Med 15:2452
Kneser U, Polykandriotis E, Ohnolz J et al (2006) Engineering of vascularized transplantable bone tissues: Induction of axial vascularization in an osteoconductive matrix using an arteriovenous loop. Tissue Eng 12:1721
Polykandriotis E, Arkudas A, Horch RE, Stürzl M, Kneser U (2007) Autonomously vascularized cellular constructs in tissue engineering: opening a new perspective for biomedical science. J Cell Mol Med 11:6
Erol OO, Spira M (1979) New capillary bed formation with a surgically constructed arteriovenous fistula. Surg Forum 30:530
Morrison WA, Dvir E, Doi K, Hurley JV, Hickey MJ, O’Brien BM (1990) Prefabrication of thin transferable axial-pattern skin flaps: an experimental study in rabbits. Br J Plast Surg 43:645
Guo L, Pribaz J (2009) Clinical flap prefabrication. Plast Reconstr Surg 124:340
Pribaz JJ, Fine NA (1994) Prelamination: defining the prefabricated flap. A case report and review. Microsurgery 15:618
Lokmic Z, Stillaert F, Morrison WA, Thompson EW, Mitchell GM (2007) An arteriovenous loop in a protected space generates a permanent, highly vascular, tissue-engineered construct. Faseb J 21:511
Rosenkilde MM, Schwartz TW (2004) The chemokine system—a major regulator of angiogenesis in health and disease. APMIS. 112:481
Maulik N, Das DK (2002) Potentiation of angiogenic response by ischemic and hypoxic preconditioning of the heart. J Cell Mol Med 6:13
Nath KA, Kanakiriya SK, Grande JP, Croatt AJ, Katusic ZS (2003) Increased venous proinflammatory gene expression and intimal hyperplasia in an aorto-caval fistula model in the rat. Am J Pathol 162:2079
Bobryshev YV, Farnsworth AE, Lord RS (2001) Expression of vascular endothelial growth factor in aortocoronary saphenous vein bypass grafts. Cardiovasc Surg 9:492
Ito A, Hirota S, Mizuno H, Kawasaki Y, Takemura T, Nishiura T, Kanakura Y, Katayama Y, Nomura S, Kitamura Y (1995) Expression of vascular permeability factor (VPF/VEGF) messenger RNA by plasma cells: possible involvement in the development of edema in chronic inflammation. Pathol Int 45:715
Dvorak HF, Detmar M, Claffey KP, Nagy JA, van de Water L, Senger DR (1995) Vascular permeability factor/vascular endothelial growth factor: an important mediator of angiogenesis in malignancy and inflammation. Int Arch Allergy Immunol 107:233
Chien S, Li S, Shyy YJ (1998) Effects of mechanical forces on signal transduction and gene expression in endothelial cells. Hypertension 31:162
Davies PF, Remuzzi A, Gordon EJ, Dewey CF Jr, Gimbrone MA Jr (1986) Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro. Proc Natl Acad Sci USA 83:2114
Asano Y, Ichioka S, Shibata M, Ando J, Nakatsuka T (2005) Sprouting from arteriovenous shunt vessels with increased blood flow. Med Biol Eng Comput 43:126
Heil M, Eitenmuller I, Schmitz-Rixen T, Schaper W (2006) Arteriogenesis versus angiogenesis: similarities and differences. J Cell Mol Med 10:45
Arkudas A, Beier JP, Heidner K, Tjiawi J, Polykandriotis E, Srour S, Sturzl M, Horch RE, Kneser U (2007) Axial prevascularization of porous matrices using an arteriovenous loop promotes survival and differentiation of transplanted autologous osteoblasts. Tissue Eng 13:1549
Fiegel HC, Pryymachuk G, Rath S, Bleiziffer O, Beier JP, Bruns H, Kluth D, Metzger R, Horch RE, Till H, Kneser U (2010) Foetal hepatocyte transplantation in a vascularized AV-Loop transplantation model in the rat. J Cell Mol Med 14:267
Mian R, Morrison WA, Hurley JV, Penington AJ, Romeo R, Tanaka Y, Knight KR (2000) Formation of new tissue from an arteriovenous loop in the absence of added extracellular matrix. Tissue Eng 6:595
Cassell OCS, Morrison WA, Messina A, Penington AJ, Thompson EW, Stevens GW, Perera JM, Kleinman HK, Hurley JV, Romeo R, Knight KR (2001) The influence of extracellular matrix on the generation of vascularized, engineered, transplantable tissue. Ann N Y Acad Sci 944:429
Manasseri B, Cuccia G, Moimas S, D’Alcontres FS, Polito F, Bitto A, Altavilla D, Squadrito F, Geuna S, Pattarini L, Zentilin L, Collesi C, Puligadda U, Giacca M, Colonna MR (2007) Microsurgical arteriovenous loops and biological templates: a novel in vivo chamber for tissue engineering. Microsurgery 27:623
Morritt AN, Bortolotto SK, Dilley RJ, Han X, Kompa AR, McCombe D, Wright CE, Itescu S, Angus JA, Morrison WA (2007) Cardiac tissue engineering in an in vivo vascularized chamber. Circulation 115:353
Bach AD, Arkudas A, Tjiawi J, Polykandriotis E, Kneser U, Horch RE, Beier JP (2006) A new approach to tissue engineering of vascularized skeletal muscle. J Cell Mol Med 10:716
Klumpp D, Horch RE, Kneser U, Beier JP (2010) Engineering skeletal muscle tissue: new perspectives in vitro and in vivo. J Cell Mol Med 14:2622
Laschke MW, Harder Y, Amon M, Martin I, Farhadi J, Ring A, Torio-Padron N, Schramm R, Rücker M, Junker D, Haüfel JM, Carvalho C, Heberer M, Germann G, Vollmar B, Menger MD (2006) Angiogenesis in tissue engineering: breathing life into constructed tissue substitutes. Tissue Eng 12:2093
Beier JP, Horch RE, Arkudas A, Polykandriotis E, Bleiziffer O, Adamek E, Hess A, Kneser U (2009) De novo generation of axially vascularized tissue in a large animal model. Microsurgery 29:42
Beier JP, Horch RE, Hess A et al (2010) Axial vascularization of a large volume calcium phosphate ceramic bone substitute in the sheep AV loop model. J Tissue Eng Regen Med 4:216
Polykandriotis E, Tjiawi J, Euler S, Arkudas A, Hess A, Brune K et al (2008) The venous graft as an effector of early angiogenesis in a fibrin matrix. Microvasc Res 75:25
Arkudas A, Pryymachuk G, Hoereth T, Beier JP, Polykandriotis E, Bleiziffer O, Horch RE, Kneser U (2009) Dose-finding study of fibrin gel-immobilized vascular endothelial growth factor 165 and basic fibroblast growth factor in the arteriovenous loop rat model. Tissue Eng Part A 15:2501
Arkudas A, Beier JP, Pryymachuk G, Hoereth T, Bleiziffer O, Polykandriotis E, Hess A, Gulle H, Horch RE, Kneser U (2010) Automatic quantitative micro-computed tomography evaluation of angiogenesis in an axially vascularized tissue-engineered bone construct. Tissue Eng Part C 16:1503
Arkudas A, Pryymachuk G, Beier JP, Weigel L, Koerner C, Singer RF, Bleiziffer O, Polykandriotis E, Horch RE, Kneser U (2012) Combination of extrinsic and intrinsic pathways significantly accelerates axial vascularization of bioartificial tissues. Plast Reconstr Surg 129:55
Beier JP, Hess A, Loew J, Heinrich J, Boos AM, Arkudas A, Polykandriotis E, Bleiziffer O, Horch RE, Kneser U (2011) De novo generation of an axially vascularized processed bovine cancellous-bone substitute in the sheep arteriovenous-loop model. Eur Surg Res 46:148
Boos AM, Loew JS, Deschler G, Arkudas A, Bleiziffer O, Gulle H, Dragu A, Kneser U, Horch RE, Beier JP (2011) Directly auto-transplanted mesenchymal stem cells induce bone formation in a ceramic bone substitute in an ectopic sheep model. J Cell Mol Med 15:1364
Boos AM, Loew JS, Weigand A, Deschler G, Klumpp D, Arkudas A, Bleiziffer O, Gulle H, Kneser U, Horch RE, Beier JP (2012) Engineering axially vascularized bone in the sheep arteriovenous-loop model. Tissue Eng Regen Med [Epub ahead of print]
Einhorn TA. Basic science of bone graft substitutes. Paper presented at the annual meeting of the orthopaedic trauma Association, october 8, 2003, salt lake city [on-line]. Available at http:\\www.hwbf.org/ota/am/ota03/bssf/OTA03BG1.htm. Accessed December 20, 2011
Friedman CD (2009) Trauma: basic principles of craniofacial bone healing and repair. In: Papel ID (ed) Facial plastic and reconstructive surgery. Thieme medical publisher, New York, p 920
Hu WW, Ward BB, Wang Z, Krebsbach PH (2010) Bone regeneration in defects compromised by radiotherapy. J Dent Res 89:77
Reuther T, Schuster T, Mende U, Kubler A (2003) Osteoradionecrosis of the jaws as a side effect of radiotherapy of head and neck tumour patients: a report of a thirty year retrospective review. Int J Oral Maxillofac Surg 32:289
Clokie C, Sandor G (2008) Reconstruction of 10 major mandibular defects using bioimplants containing BMP-7. J Can Dent Assoc 74:67
Schuckert K, Jopp S, Teoh S (2009) Mandibular defect reconstruction using three-dimensional polycaprolactone scaffold in combination with platelet-rich plasma and recombinant human bone morphogenetic protein-2: de novo synthesis of bone in a single case. Tissue Eng Part A 15:493
Elshahat A (2006) Correction of craniofacial skeleton contour defects using bioactive glass particles. Egypt J Plast Reconstr Surg 30:113
Trautvetter W, Kaps C, Schmelzeisen R, Sauerbier S, Sittinger M (2011) Tissue-engineered polymer-based periosteal bone grafts for maxillary sinus augmentation: five-year clinical results. J Oral Maxillofac Surg 69:2753
Yazar S (2007) Selection of recipient vessels in microsurgical free tissue reconstruction of head and neck defects. Microsurgery 27:588
Eweida A, Nabawi A, Marei M, Khalil MR, Elhammady H (2011) Mandibular reconstruction using an axially vascularized tissue-engineered construct. ASIR 5:2
The Guide for the Care and Use of Laboratory Animals., Published by the National Institutes of Health (NIH publication No. 85-23, revised 1996)
Viateeau V, Avramoglou D, Guillemin G et al (2008) Sourcebook of models for biomedical research. In: Conn PM (ed) Animal models for bone tissue engineering purposes. Humana press, New Jersey, pp 725–763
Daculsi G, LeGeros RZ, Nery E, Lynch K, Kerebel B (1989) Transformation of biphasic calcium phosphate ceramics in vivo: ultrastructural and physicochemical characterization. J Biomed Mater Res 23:883
Daculsi G, LeGeros RZ, Heughebaert M, Barbieux I (1990) Formation of carbonate-apatite crystals after implantation of calcium phosphate ceramics. Calcif Tissue Int 6:20
de Oliveira RS, Brigato R, Madureira JF, Cruz AA, de Mello Filho FV, Alonso N, Machado HR (2007) Reconstruction of a large complex skull defect in a child: a case report and literature review. Childs Nerv Syst 23:1097
Lobo SE, Arinzeh TL (2010) Biphasic Calcium Phosphate Ceramics for Bone Regeneration and Tissue Engineering Applications. Materials 3:815
Kuhne JH, Bartl R, Frisch B et al (1994) Bone formation in coralline hydroxyapatite. Effects of pore size studied in rabbits. Acta Ortop Scand 65:246
Druecke D, Langer S, Lamme E, Pieper J, Garkovic M, Steinau HU, Homann HH (2004) Neovascularization of polyether ester block-copolymer scaffolds in vivo: long term investigations using intravital fluorescent microscopy. J Biomed Mater Res 68:10
Menger MD, Hammersen F, Walter P, Messmer K (1990) Neovascularization of prosthetic vascular grafts. Quantitative analysis of angiogenesis and microhemodynamics by means of intravital microscopy. Thorac Cardiovasc Surg 38:139
Marx RE (2001) Platelet-rich plasma (PRP): what is PRP and what is not PRP? Implant Dent 10:225
Eppley B, Pietrzak W, Blanton M (2006) Platelet-Rich Plasma: A Review of Biology and Applications in Plastic Surgery. Plastic Reconstructive Surgery 118:147
Whitman DH, Berry RL, Green DM (1997) Platelet gel: an autologous alternative to fibrin glue with applications in oral and maxillofacial surgery. J Oral Maxillofac Surg 55:1294
Freymiller EG, Aghaloo TL (2004) Platelet-rich plasma: ready or not? J Oral Maxillofac Surg 62:484
Plachokova AS, Nikolidakis D, Mulder J, Jansen JA, Creugers NH (2008) Effect of platelet-rich plasma on bone regeneration in dentistry: a systematic review. Clin Oral Implants Res 19:539
Nikolidakis D, Jansen JA (2008) The biology of platelet-rich plasma and its application in oral surgery: literature review. Tissue Eng Part B 14:249
Hu ZM, Peel SA, Ho SK, Sándor GK, Clokie CM (2009) Comparison of platelet-rich plasma, bovine BMP, and rhBMP-4 on bone matrix protein expression in vitro. Growth Factors 27:280
Urist MR (1965) Bone: formation by autoinduction. Science 150:893
Raida M, Heymann AC, Günther C, Niederweiser D (2006) Role of bone morphogenetic protein 2 in the crosstalk between endothelial progenitor cells and mesenchymal stem cells. Int J Mol Med 18:735
Cenni E, Avnet S, Fotia C, Salerno M, Baldrini N (2010) Platelet-rich plasma impairs osteoclast generation from human precursors of peripheral blood. J Orthop Res 28:792
Park EJ, Kim ES, Weber HP, Wright RF, Mooney DJ (2008) Improved bone healing by angiogenic factor-enriched platelet-rich plasma and its synergistic enhancement by bone morphogenetic protein-2. Int J Oral Maxillofac Implants 23:818
Schuckert KH, Jopp S, Osadnik M (2010) Modern bone regeneration instead of bone transplantation: a combination of recombinant human bone morphogenetic protein-2 and platelet-rich plasma for the vertical augmentation of the maxillary bone, a single case report. Tissue Eng Part C 16:1335
Meyer RA Jr, Gruber HE, Howard BA, Tabor OB Jr, Murakami T, Kwiatkowski TC, Wozney JM, Hanley EN Jr (1999) Safety of recombinant human bone morphogenetic protein-2 after spinal laminectomy in the dog. Spine 24:747
McKay B, Sandhu HS (2002) Use of recombinant human bone morphogenetic protein-2 in spinal fusion applications. Spine 27:66
Hori Y, Tamai S, Okuda H et al (1979) Blood vessel transplantation to bone. J Hand Surg Am 4:23
Bochud RC, Büchler U (1994) Kienböck’s disease, early stage 3-height reconstruction and core revascularization of the lunate. J Hand Surg Br 19:466
Tamai S, Yajima H, Ono H (1993) Revascularization procedures in the treatment of Kienböck’s disease. Hand Clin 9:455
Akita S, Tamai N, Myoui A, Nishikawa M, Kaito T, Takaoka K, Yoshikawa H (2004) Capillary vessel network integration by inserting a vascular pedicle enhances bone formation in tissue-engineered bone using interconnected porous hydroxyapatite ceramics. Tissue Eng 10:789
Kloeters O, Berger I, Ryssel H, Megerle K, Leimer U, Germann G (2011) Revitalization of cortical bone allograft by application of vascularized scaffolds seeded with osteogenic induced adipose tissue derived stem cells in a rabbit model. Arch Orthop Trauma Surg 131(10):1459–1466
Lovett M, Lee K, Edwards A, Kaplan D (2009) Vascularization strategies for tissue engineering. Tissue Eng 15:353
Warnke P, Springer I, Wiltfang J, Acil Y, Eufinger H, Wehmöller M, Russo P, Bolte H, Sherry E, Behrens E, Terheyden H (2004) Growth and transplantation of a custom vascularised bone graft in a man. Lancet 364:766
Acknowledgments
The mandibular reconstruction study was funded by a research grant from the University of Alexandria (Alexandria University Research Enhancement Program; Alex REP, code HLTH-09). The authors would like to acknowledge with great appreciation the team of Abou Elnaga Laboratories in Alexandria, namely Dr. Sahdy Foad for help with the PRP protocol standardization. The animal experiments were approved by the ethics committee of the University of Alexandria and complied with the current laws of the local governmental authorities.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eweida, A.M., Nabawi, A.S., Elhammady, H.A. et al. Axially vascularized bone substitutes: a systematic review of literature and presentation of a novel model. Arch Orthop Trauma Surg 132, 1353–1362 (2012). https://doi.org/10.1007/s00402-012-1550-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-012-1550-3