Abstract
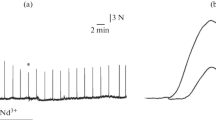
The protective effects of various divalent cations against the irreversible damage of myocardium, a phenomenon termed “the Ca2+-paradox”, were examined in the isolated perfused pigeon heart. All cations examined were added at a concentration of 200 μmol l−1 in the “calcium-free” medium. In hearts perfused with low calcium, upon normal calcium repletion, the maximal recovery of the contractile tension (in the 2nd minute) was approximately 115% and the recovery obtained at the end of reperfusion was 81.5% (compared to the equilibration period value). From the other divalent cations examined, the presence of cobalt, nickel, manganese or barium during calcium depletion powerfully protected the pigeon heart. Upon calcium repletion, the maximal recovery of contractile tension was approximately 60%, 76.5%, 100% and 85%, the recovery estimated at the end of reperfusion was 40%, 12%, 70% and 53%, and the resting tension estimated at the end of reperfusion was 2.69±0.18 g, 6.40±0.50 g, 1.20±0.10 g and 1.90±0.10 g for cobalt, nickel, manganese and barium, respectively. On the contrary, strontium exerted no protective effects. The protective effects were also indicated by reduced total protein and lactate dehydrogenase activity release into the effluent perfusate and maintenance of electrical activity. The effectiveness of the added divalent cations (with the exception of strontium) showed a strong dependence upon their ionic radius. The most potent inhibitors of this phenomenon in the pigeon heart were the divalent cations having an ionic radius closer to the ionic radius of calcium. These results are discussed in terms of the possible mechanisms involved in the protective effects of these cations.








Similar content being viewed by others
References
Alto LE, Dhalla NS (1979) Myocardial cation contents during induction of the calcium paradox. Am J Physiol 273:H713–H719
Alto LE, Elimban V, Lukas A, Dhalla NS (2000) Modification of heart sarcolemmal Na+/K+-ATPase activity during development of the calcium paradox. Mol Cell Biochem 207:87–94
Baker JE, Hearse DJ (1983) Slow calcium channel blockers and the calcium paradox: comparative studies in the rat with seven drugs. J Mol Cell Cardiol 15:475–485
Bers DM, Langer GA (1979) Uncoupling effects on cardiac contractility and sarcolemmal Ca2+ binding. Am J Physiol 237:H332–H341
Bers DM, Phillipson KD, Nishimoto AU (1980) Sodium-calcium exchange and sideness of isolated sarcolemmal vesicles. Biochim Biophys Acta 601:358–371
Bhojani IH, Chapman RA (1990) The effect of bathing sodium ions upon the intracellular sodium activity in calcium paradox of isolated ferret ventricular muscle. J Mol Cell Cardiol 22:507–522
Bosteels S, Matejovic P, Flameng W, Mubagwa K (1999) Sodium influx via a non-selective pathway activated by the removal of extracellular divalent cations: possible role in the calcium paradox. Cardiovasc Res 43:417–425
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilising the principle of protein-dye binding. Anal Biochem 72:248–254
Chapman RA, Tunstall J (1987) The calcium paradox of the heart. Prog Biophys Mol Biol 50:67–96
Chapman RA, Suleiman MS, Rodrigo GC, Tunstall J (1991) The calcium paradox: a role for [Na]I, a cellular or tissue basis a property unique to the Langendorff perfused heart? A bundle of contradictions. J Mol Cell Cardiol 23:773–777
Dhalla NS, Alto LE, Singal PK (1983) Role of Na+/Ca2+ exchange in the development of cardiac abnormalities due to calcium paradox. Eur Heart J 4:51–56
Frank JS, Langer GA, Nudd LM, Seraydarian K (1977) The myocardial cell surface: its histochemistry and the effects of sialic acid and calcium removal on structure and ionic exchange. Circ Res 41:702–714
Gaitanaki C, Anezaki M, Margieti MM, Papazafiri P, Beis I (2002) Characterisation of the calcium paradox in the isolated perfused pigeon heart: protection by hypothermia, acidosis and alkalosis. Cell Physiol Biochem 12:93–100
Gaitanaki C, Papazafiri P, Beis I (2003) The calpain-calpastatin system and the calcium paradox in the isolated perfused pigeon heart. Cell Physiol Biochem 13:173–180
Goshima K, Wakabayashi S, Masuda A (1980) Ionic mechanisms of morphological changes of cultured myocardial cells on successive incubation with media without and with Ca2+. J Mol Cell Cardiol 7:1135–1157
Grinwald PM, Nayler WG (1981) Calcium entry in the calcium paradox. J Mol Cell Cardiol 13:867–880
Guarnieri T (1988) Decrease in the transmembrane sodium activity gradient in ferret papillary muscle as a prerequisite to the calcium paradox. J Clin Invest 81:1938–1944
Harding RJ, Duncan CJ (1997) Protection against cellular damage in the perfused rat heart by lowered pH. Eur J Pharmacol 330:47–53
Harrow JAC, Das PK, Dhalla NS (1978) Influence of some divalent cations on heart sarcolemmal bound enzymes and calcium binding. Biochem Pharmacol 27:2605–2609
Hunter DR, Haworth RA, Berkoff HA (1981) Cellular manganese uptake by the isolated perfused rat heart: a probe for sarcolemma calcium channel, J Mol Cell Cardiol 13:823–832
Jansen MA, Van Echteld CJA, Ruigrok TJC (1998) Na+/Ca2+ exchange during Ca2+ repletion is not prerequisite for the Ca2+ paradox in isolated rat hearts. Pflugers Arch 436:515–520
Kohlhard M, Haap K (1980) On the mechanism underlying the cobalt-induced inhibition of slow inward current in mammalian ventricular myocardium. J Mol Cell Cardiol 12:1075–1090
Lemasters JJ (1999) The mitochondrial permeability transition and the calcium, oxygen and pH paradoxes: one paradox after another. Cardiovasc Res 44:470–473
Luckacs GL, Fonyo A (1986) The Ba2+ sensitivity of the Na+-induced Ca2+ efflux in heart mitochondria. The site of inhibitory action. Biochim Biophys Acta 858:125–134
Macianskiene R, Matejovic P, Sipido K, Flameng W, Nubagwa K (2001) Modulation of the extracellular divalent cation-inhibited non-selective conductance in cardia cells by metabolic inhibition and by oxidants. J Mol Cell Cardiol 33:1371–1385
Nayler WG (1988) Calcium antagonists. Academic Press, London
Nayler WG, Grinwald PM (1982) Dissociation of Ca2+ accumulation from protein release in Ca-paradox: effect of barium. Am J Physiol 242:H203–H210
Nayler WG, Perry SE, Daly MJ (1983) Cobalt, manganese and the calcium paradox. J Mol Cell Cardiol 15:735–747
Ochi R (1975) Manganese action potentials in mammalian cardiac muscle. Experientia 31:1048–1049
Oksental AN, Jynge P (1986) Myocardial protection by micromolar manganese in the calcium paradox and additive effects of verapamil. Basic Res Cardiol 81:581–593
Persad S, Vrbanova A, Meij JTA, Panagia V, Dhalla NS (1993) Possible role of phospholipase C in the induction of the Ca2+ paradox in the heart. Mol Cell Biochem 121:181–190
Reuter H (1979) Properties of two inward membrane currents in the heart. Annu Rev Physiol 41:413–424
Rich TL, Langer GA (1982) Calcium depletion in rabbit myocardium: calcium paradox protection by hypothermia and cation substitution. Circ Res 51:131–141
Ruirgok TJC (1990) Is an increase of intracellular Na+ during Ca2+ depletion essential for the occurrence of the calcium paradox? J Mol Cell Cardiol 22:499–501
Schwartz A, Lindenmeyer GE, Allen JC (1975) The sodium-potassium adenosine triphosphate: pharmacological, physiological and biochemical aspects. J Pharmacol Rev 27:3–134
Smith FM, West NH, Jones DR (2000) The cardiovascular system. In: Whittow GC (ed) Sturkie’s avian physiology, 5th edn. Academic Press, pp 141–231
Touraki M, Beis I (1991) Protective effects of manganese, cobalt, nickel, and barium against a calcium paradox in the isolated frog heart. J Exp Zool 259:287–293
Touraki M, Lazou A (1992) Protective effect of adenosine against a calcium paradox in the isolated frog heart. Can J Physiol Pharmacol 70:115–120
Trautein W, Cavalie A (1985) Cardiac calcium channels and their control by neurotransmitters and drugs. J Am Cell Cardiol 6:1401–1416
Van Echteld CJA, Kirkels JH, Eijgelshoven MHJ, Van der Meer P, Ruigrok TJC (1991) Intracellular sodium during ischaemia and calcium-free perfusion: a 23Na NMR study. J Lom Cell Cardiol 23:297–307
Volkman R, Winell S, Erickson D (1986) Effect of cyanide and verapamil on sodium free contractures in the frog heart. Comp Biochem Physiol 84C:247–255
Ward CW, Castro GA, Falbrain D (1969) Carbon dioxide fixation and phosphoenolpyruvate metabolism in Trichinella spiralis larvae. J Parasitol 55:67–71
Zimmerman ANE, Hulsmann WC (1966) Paradoxical influence of calcium ions on the permeability of the cell membranes of the isolated rat heart. Nature 211:646–647
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by: G. Heldmaier
Rights and permissions
About this article
Cite this article
Gaitanaki, C., Labrakakis, C., Papazafiri, P. et al. Various divalent cations protect the isolated perfused pigeon heart against a calcium paradox. J Comp Physiol B 174, 371–382 (2004). https://doi.org/10.1007/s00360-004-0423-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-004-0423-7