Abstract
Objective
Phosphodiesterases (PDEs) play a role in controlling cyclic nucleotide action, including cyclic guanosine monophosphate (cGMP). Previous studies have ascribed a protective role of cGMP signaling on hypoxia-mediated cancer progression. Herein, we determine their potential role in hypoxia-mediated chemoresistance and immune escape.
Materials and Methods
Phosphodiesterase assays were used to measure PDE activity in prostate cancer cell lines (DU145, PC3). Immunoblots were performed to determine the presence of PDEs in human prostate tissue samples. The effect of PDE inhibition on hypoxia-induced chemoresistance (compared to normoxic controls, 20% O2) was determined using clonogenic assays. Flow cytometry was used to determine the effects of PDE inhibition on surface MHC class I-related chain A (MICA), a natural killer (NK) cell-activating ligand. A mouse model was used to evaluate the in vivo effects of PDE inhibition on the growth of human prostate cancer cells.
Results
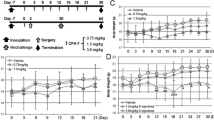
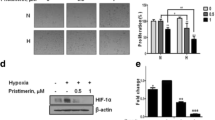
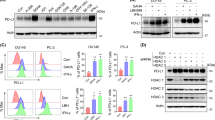
PDE5 and PDE11 were the most prominent PDEs in the cell lines, representing between 86 and 95% of the total cGMP-specific PDE activity. Treatment of DU-145 cells with a PDE inhibitor significantly reduced the hypoxia-associated acquisition of resistance to doxorubicin, with a mean 51% reduction in surviving fraction compared to controls (p < 0.001, ANOVA). As well, PDE inhibition completely reversed (p = 0.02, ANOVA) hypoxia-induced shedding of the immune stimulatory molecule, MICA, and attenuated the growth of human prostate tumor xenografts in an NK cell-competent murine model (p = 0.03, Wilcoxon, Mann–Whitney).
Conclusions
These results suggest a rationale for future studies on the potential therapeutic applications of PDE inhibitors in men with prostate cancer.



Similar content being viewed by others
Abbreviations
- BPH:
-
Benign prostatic hyperplasia
- cGMP:
-
Cyclic guanosine monophosphate
- cAMP:
-
Cyclic adenosine monophosphate
- MICA:
-
MHC class I-related chain A
- PDE:
-
Phosphodiesterase
- PSA:
-
Prostate-specific antigen
References
Semenza GL (2002) HIF-1 and tumor progression: pathophysiology and therapeutics. Trends Mol Med 8(4 suppl):S62–S67
Movsas B, Chapman JD, Greenberg RE et al (2000) Increasing levels of hypoxia in prostate carcinoma correlate significantly with increasing clinical stage and patient age: and Eppendorf pO(2) study. Cancer 89:2018–2024
Gillet J-P, Gottesmon MM (2010) Multi-drug resistance in cancer. Methods Mol Biol 596:47–76
Frederiksen LJ, Siemens DR, Heaton JP et al (2003) Hypoxia induced resistance to doxorubicin in prostate cancer cells is inhibited by low concentrations of glyceryl trinitrate. J Urol 170:1003–1007
Matthews NE, Adams MA, Maxwell LR et al (2001) Nitric oxide-mediated regulation of chemosensitivity in cancer cells. J Natl Cancer Inst 93:1879–1885
Wartenberg M, Gronczynska S, Bekhite MM et al (2005) Regulation of the multidrug resistance transporter P-glycoprotein in multicellular prostate tumor spheroids by hyperthermia and reactive oxygen species. Int J Cancer 113:229–240
Siemens DR, Hu N, Sheikhi AK et al (2008) Hypoxia increases tumor cell shedding of MHC class I chain-related molecule: role of nitric oxide. Cancer Res 68:4746–4753
Barsoum IB, Hamilton TK, Li X, Cotechini T, Miles EA, Siemens DR, Graham CH (2011) A mechanism of hypoxia-induced immune escape in cancer cells. Cancer Res 71:7433–7441
Groh V, Wu J, Yee C, Spies T (2002) Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 419:734–738
Frederiksen LJ, Sullivan R, Maxwell LR et al (2007) Chemosensitization of cancer in vitro and in vivo by nitric oxide signaling. Clin Cancer Res 13(7):2199–2206
Bell EN, Tse MY, Frederiksen LJ et al (2007) Atrial natriuretic peptide attenuates hypoxia induced chemoresistance in prostate cancer cells. J Urol 177(2):751–756
Yasuda H, Nakayama K, Watanabe M et al (2006) Nitroglycerin treatment may enhance chemosensitivity to docetaxel and carboplatin in patients with lung adenocarcinoma. Clin Cancer Res 12(22):6748–6757
Siemens DR, Heaton JP, Adams MA et al (2009) Phase II study of nitric oxide donor for men with increasing prostate-specific antigen level after surgery or radiotherapy for prostate cancer. Urology 74(4):878–883
Soderling SH, Beavo JA (2000) Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Curr Opin Cell Biol 12(2):174–179
Bender AT, Beavo JA (2006) Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58(3):488–520
Abdollahi M, Chan TS, Subrahmanyam V et al (2003) Effects of phosphodiesterase 3,4,5 inhibitors on hepatocyte cAMP levels, glycogenolysis, gluconeogenesis and susceptibility to a mitochondrial toxin. Mol Cell Biochem 252(1–2):205–211
Sarfati M, Mateo V, Baudet S et al (2003) Sildenafil and vardenafil, types 5 and 6 phosphodiesterase inhibitors, induce caspase-dependent apoptosis of B-chronic lymphocytic leukemia cells. Blood 101(1):265–269
Zhu B, Vemavarapu L, Thompson WJ et al (2005) Suppression of cyclic GMP-specific phosphodiesterase 5 promotes apoptosis and inhibits growth in HT29 cells. J Cell Biochem 94(2):336–350
Qian C-N, Takahashi M, Kahnoski RJ, The BT (2003) Effect of sildenafil citrate on an orthotopic prostate cancer growth and metastasis model. J Urol 170:994–997
Das A, Durrant D, Mitchell C (2010) Sildenafil increases chemotherapeutic efficacy of doxorubicin in prostate cancer and ameliorates cardiac dysfunction. Proc Natl Acad Sci USA 107(42):18202–18207
Davis CW, Daly JW (1979) A simple direct assay of 3′,5’-cyclic nucleotide phosphodiesterase activity based on the use of polyacrylamide-bononate affinity gel chromatography. J Cyclic Nucleotide Res 5(1):65–74
Tannock IF, de Wit R, Berry WR et al (2004) Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 351:1502–1512
Petrylak DP, Tangen CM, Hussain MH et al (2004) Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 351(15):1513–1520
Pienta KJ, Naik H, Lehr JE (1996) Effect of estramustine, etoposide, and taxol on prostate cancer cell growth in vitro and in vivo. Urology 48:164–170
Cengiz E, Karaca B, Kucukzeybek Y et al (2010) Overcoming drug resistance in hormone- and drug-refractory prostate cell line, PC-3 by docetaxel and gossypol combination. Mol Biol Rep 37:1269–1277
Wu JD, Higgins LM, Steinle A et al (2004) Prevalent expression of the immunostimulatory MHC class I chain-related molecule is counteracted by shedding in prostate cancer. J Clin Invest 114:560–568
Holdenrieder S, Stieber P, Peterfi A et al (2006) Soluble MICA in malignant diseases. Int J Cancer 118:684–687
Marten A, Lilienfeld-Toal M, Buchler MW et al (2006) Soluble MIC is elevated in the serum of patients with pancreatic carcinoma diminishing γδ T cell cytotoxicity. Int J Cancer 119:2359–2365
Acknowledgments
We gratefully acknowledge the financial support of Prostate Cancer Canada and Canadian Institutes of Health Research.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hamilton, T.K., Hu, N., Kolomitro, K. et al. Potential therapeutic applications of phosphodiesterase inhibition in prostate cancer. World J Urol 31, 325–330 (2013). https://doi.org/10.1007/s00345-012-0848-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-012-0848-7