Abstract
Purpose
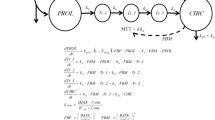
Trastuzumab emtansine (T-DM1) is an antibody-drug conjugate in the development for the treatment of human epidermal growth factor receptor 2-positive cancers. Thrombocytopenia (TCP) is the dose-limiting toxicity of T-DM1. A semimechanistic population pharmacokinetic/pharmacodynamic (PK/PD) model was developed to characterize the effect of T-DM1 on patient platelet counts.
Methods
A PK/PD model with transit compartments that mimic platelet development and circulation was fit to concentration-platelet–time course data from two T-DM1 single-agent studies (TDM3569g; N = 52 and TDM4258g; N = 112). NONMEM® 7 software was used for model development. Data from a separate phase II study (TDM4374g; N = 110) were used for model evaluation. Patient baseline characteristics were evaluated as covariates of model PD parameters.
Results
The model described the platelet data well and predicted the incidence of grade ≥3 TCP. The model predicted that with T-DM1 3.6 mg/kg given every 3 weeks (q3w), the lowest platelet nadir would occur after the first dose. Also predicted was a patient subgroup (46 %) having variable degrees of downward drifting platelet–time profiles, which were predicted to stabilize by the eighth treatment cycle to platelet counts above grade 3 TCP. Baseline characteristics were not significant covariates of PD parameters in the model.
Conclusions
This semimechanistic PK/PD model accurately captures the cycle 1 platelet nadir, the downward drift noted in some patient platelet–time profiles, and the ~8 % incidence of grade ≥3 TCP with T-DM1 3.6 mg/kg q3w. This model supports T-DM1 3.6 mg/kg q3w as a well-tolerated dose with minimal dose delays or reductions for TCP.





Similar content being viewed by others
References
Lewis Phillips GD, Li G, Dugger DL et al (2008) Targeting HER2-positive breast cancer with trastuzumab emtansine, an antibody-cytotoxic drug conjugate. Cancer Res 68:9280–9290. doi:10.1158/0008-5472.CAN-08-1776
Krop IE, Beeram M, Modi S et al (2010) Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol 28:2698–2704. doi:10.1200/JCO.2009.26.2071
Burris HA III, Rugo H, Vukelja SJ et al (2011) Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol 29:398–405. doi:10.1200/JCO.2010.29.5865
Krop I, LoRusso P, Miller KD et al (2010) A phase II study of trastuzumab-DM1 (T-DM1), a novel HER2 antibody-drug conjugate, in patients with HER2+ metastatic breast cancer who were previously treated with an anthracycline, a taxane, capecitabine, lapatinib, and trastuzumab. Presented at European Society for Medical Oncology Congress, October 8–12, Milan, Italy (abstract 277O)
Gupta M, LoRusso PM, Wang B et al (2011) Clinical implications of pathophysiological and demographic covariates on the population pharmacokinetics of trastuzumab emtansine, a HER2-targeted antibody-drug conjugate, in patients with HER2-positive metastatic breast cancer. J Clin Pharmacol. doi:10.1177/0091270011403742
Friberg LE, Henningsson A, Maas H, Nguyen L, Karlsson MO (2002) Model of chemotherapy-induced myelosuppression with parameter consistency across drugs. J Clin Oncol 20:4713–4721. doi:10.1200/JCO.2002.02.140
Friberg LE, Karlsson MO (2003) Mechanistic models for myelosuppression. Invest New Drugs 21:183–194
van Kesteren C, Zandvliet AS, Karlsson MO et al (2005) Semi-physiological model describing the hematological toxicity of the anti-cancer agent indisulam. Invest New Drugs 23:225–234
Latz JE, Rusthoven JJ, Karlsson MO, Ghosh A, Johnson RD (2006) Clinical application of a semimechanistic-physiologic population PK/PD model for neutropenia following pemetrexed therapy. Cancer Chemother Pharmacol 57:427–435. doi:10.1007/s00280-005-0035-2
Joerger M, Huitema AD, Richel DJ et al (2007) Population pharmacokinetics and pharmacodynamics of paclitaxel and carboplatin in ovarian cancer patients: a study by the European organization for research and treatment of cancer-pharmacology and molecular mechanisms group and new drug development group. Clin Cancer Res 13:6410–6418. doi:10.1158/1078-0432.CCR-07-0064
Schmitt A, Gladieff L, Laffont C et al (2010) Factors for hematopoietic toxicity of carboplatin: refining the targeting of carboplatin systemic exposure. J Clin Oncol 28:4568–4574. doi:10.1200/JCO.2010.29.3597
Gupta P, Friberg LE, Karlsson MO, Krishnaswami S, French J (2010) A semi-mechanistic model of CP-690,550-induced reduction in neutrophil counts in patients with rheumatoid arthritis. J Clin Pharmacol 50:679–687. doi:10.1177/0091270009346060
Beal SL, Boeckman AJ, Sheiner LB (1992) NONMEM users guide, part IV. Regents of California, San Francisco. ftp://nonmem.iconplc.com/Public/nonmem720/guides/iv.pdf. Accessed 16 Dec 2011
Khandelwal A, Harling K, Jonsson EN, Hooker A, Karlsson M (2011) A fast method for testing covariates in population PK/PD models. AAPSJ 13:464–472. doi:10.1208/s12248-011-9289-2
Hurvitz SA, Dirix L, Kocsis J et al (2011) Trastuzumab emtansine (T-DM1) versus trastuzumab + docetaxel in previously untreated HER2-positive metastatic breast cancer (MBC): primary results of a randomized, multicenter, open-label phase II study (TDM4450g/BO21976). Presented at European Multidisciplinary Cancer Congress, September 23–27, Stockholm, Sweden (abstract 5001)
Perry MC, McKinney MF (2008) Chemotherapeutic agents: trastuzumab (herceptin). In: Perry MC (ed) The chemotherapy source book, 4th edn. Lippincott Williams & Wilkins, Philadelphia, p 629
Blum RH, Kahlert T (1978) Maytansine: a phase I study of an ansa macrolide with antitumor activity. Cancer Treat Rep 62:435–438
Blum RH, Wittenberg BK, Canellos GP et al (1978) A therapeutic trial of maytansine. Cancer Clin Trials 1:113–117
Rodon J, Garrison M, Hammond LA et al (2008) Cantuzumab mertansine in a three-times a week schedule: a phase I and pharmacokinetic study. Cancer Chemother Pharmacol 62:911–919. doi:10.1007/s00280-007-0672-8
Tolcher AW, Ochoa L, Hammond LA et al (2003) Cantuzumab mertansine, a maytansinoid immunoconjugate directed to the CanAg antigen: a phase I, pharmacokinetic, and biologic correlative study. J Clin Oncol 21:211–222. doi:10.1200/JCO.2003.05.137
Galsky MD, Eisenberger M, Moore-Cooper S et al (2008) Phase I trial of the prostate-specific membrane antigen-directed immunoconjugate MLN2704 in patients with progressive metastatic castration-resistant prostate cancer. J Clin Oncol 26:2147–2154. doi:10.1200/JCO.2007.15.0532
O’Shaughnessy JA, Venzon DJ, Gossard M et al (1995) A phase I study of sequential versus concurrent interleukin-3 and granulocyte-macrophage colony-stimulating factor in advanced breast cancer patients treated with FLAC (5-fluorouracil, leucovorin, doxorubicin, cyclophosphamide) chemotherapy. Blood 86:2913–2921
Maze R, Moritz T, Williams DA (1994) Increased survival and multilineage hematopoietic protection from delayed and severe myelosuppressive effects of a nitrosourea with recombinant interleukin-11. Cancer Res 54:4947–4951
National Cancer Institute (2006) Common terminology criteria for adverse events v3.0 (CTCAE). http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed 20 June 2011
Acknowledgments
The study was funded by Genentech, Inc. Support for third-party writing assistance was provided by Genentech, Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bender, B.C., Schaedeli-Stark, F., Koch, R. et al. A population pharmacokinetic/pharmacodynamic model of thrombocytopenia characterizing the effect of trastuzumab emtansine (T-DM1) on platelet counts in patients with HER2-positive metastatic breast cancer. Cancer Chemother Pharmacol 70, 591–601 (2012). https://doi.org/10.1007/s00280-012-1934-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-012-1934-7