Abstract
Background
Altered FDG uptake patterns were noted in certain lymphoblastic lymphoma patients during therapy.
Objective
To describe these altered FDG uptake patterns and their relationship to chemotherapy.
Materials and methods
Thirty-five FDG PET or PET/CT scans obtained in 11 children with lymphoblastic lymphoma were retrospectively reviewed. FDG uptake patterns were recorded. SUV measurements were performed in liver and facial soft tissues. Results were correlated with induction chemotherapy regimens.
Results
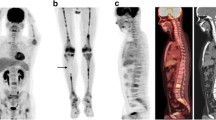
Six of the children had transiently altered FDG uptake with increased uptake in the superficial soft tissues, most notably involving the face. Altered uptake was noted approximately 1 month after initiation of chemotherapy and subsequently resolved. Hepatic uptake was transiently reduced on the 1-month scan in all six children with increased facial uptake. No significant FDG uptake in lymphoma was seen on five of six scans with altered uptake; however, two of these five affected children had FDG uptake in lymphoma on the next follow-up examination. Blood glucose levels in the affected children were in the normal range. All six children with altered FDG uptake received the same induction chemotherapy regimen, which included very high doses of corticosteroids.
Conclusions
Children with lymphoblastic lymphoma on induction chemotherapy protocols including very high doses of corticosteroids transiently demonstrated altered FDG uptake patterns, including increased superficial facial uptake and reduced hepatic uptake. The facial uptake is probably the FDG PET equivalent of Cushingoid facies. Caution in interpreting scans with this altered FDG uptake pattern is suggested, as uptake at sites of lymphomatous involvement may potentially be affected.





Similar content being viewed by others
References
Shankar A, Fiumara F, Pinkerton R (2008) Role of FDG PET in the management of childhood lymphomas—case proven or is the jury still out? Eur J Canc 44:663–673
Raef R, Omar W, Kotb M et al (2010) Role of PET/CT in malignant pediatric lymphoma. Eur J Nucl Med Mol Imag 37:319–329
Jacene HA, Ishimori T, Engles JM et al (2006) Effects of pegfilgrastim on normal biodistribution of 18F-FDG: preclinical and clinical studies. J Nucl Med 47:950–956
Kazama T, Swanston N, Podoloff DA et al (2005) Effect of colony-stimulating factor and conventional- or high-dose chemotherapy on FDG uptake in bone marrow. Eur J Nucl Med Mol Imag 32:1406–1411
Sugawara Y, Fisher SJ, Zasadny KR et al (1998) Preclinical and clinical studies of bone marrow uptake of fluorine-1-fluorodeoxyglucose with or without granulocyte colony-stimulating factor during chemotherapy. J Clin Oncol 16:173–180
Yamane T, Daimaru O, Ito S et al (2004) Decreased 18F-FDG uptake 1 day after initiation of chemotherapy for malignant lymphomas. J Nucl Med 45:1838–1842
Ramadori G, Cameron S (2010) Effects of systemic chemotherapy on the liver. Ann Hepatol 9:133–143
Matsumoto T, Yamasaki S, Arakawa A et al (2007) Exposure to a high total dosage of glucocorticoids produces non-alcoholic steatohepatitis. Pathol Int 57:388–389
Shigematsu A, Okada K, Abe N et al (2006) Non-alcoholic steatohepatitis occurring in a patient with T-lymphoblastic lymphoma during chemotherapy including prednisolone. Leuk Lymphoma 47:1397–1399
Candelli M, Nista EC, Pignataro G et al (2003) Steatohepatitis during methylprednisolone therapy for ulcerative colitis. J Intern Med 253:391–392
Dourakis SP, Sevastianos VA, Kaliopi P (2002) Acute severe steatohepatitis related to prednisolone therapy. Am J Gastroenterol 97:1074–1075
Abele JT, Fung CI (2010) Effect of hepatic steatosis on liver FDG uptake measured in mean standard uptake values. Radiology 254:917–924
Rabkin Z, Israel O, Zohar K (2010) Do hyperglycemia and diabetes affect the incidence of false-negative 18F-FDG PET/CT studies in patients evaluated for infection or inflammation and cancer? A comparative analysis. J Nucl Med 51:1015–1020
Diederichs CG, Staib L, Glatting G et al (1998) FDG PET: elevated plasma glucose reduces both uptake and detection rate of pancreatic malignancies. J Nucl Med 39:1030–1033
Torizuka T, Zasadny KR, Wahl RL (1999) Diabetes decreases FDG accumulation in primary lung cancer. Clin Positron Imaging 2:281–287
Chang YC, Yen TC, Ng KK et al (2005) Does diabetes mellitus influence the efficacy of FDG-PET in the diagnosis of cervical cancer? Eur J Nucl Med Mol Imag 32:647–652
Gorenberg M, Hallett WA, O’Doherty MJ (2002) Does diabetes affect [18F]FDG standardized uptake values in lung cancer? Eur J Nucl Med Mol Imag 29:1324–1327
Moreno-Fernandez J, Gutierrez-Alcantara C, Galvez Moreno A et al (2008) Corticotrophin-dependent Cushing syndrome due to sacrococcygeal teratoma detected by [18F]fluorodeoxyglucose positron emission tomography. J Clin Endocrinol Metab 93:3282–3283
Cho S, Ra YJ, Lee CT et al (2008) Difficulties in diagnosis and management of ectopic Cushing syndrome. J Thorac Oncol 3:444–446
Shah NA, Urusova IA, D’Agnolo A et al (2007) Primary hepatic carcinoid tumor presenting as Cushing’s syndrome. J Endocrinol Invest 30:327–333
Hashiba T, Saitoh Y, Asanuma N et al (2006) Reduction of a pancreatic tumor after total removal of an ACTH secreting pituitary tumor: differential diagnosis of Cushing’s syndrome. Endocr J 53:203–208
Fasshauer M, Lincke T, Witzigmann H et al (2006) Ectopic Cushing syndrome caused by a neuroendocrine carcinoma of the mesentery. BMC Canc 6:108
Kumar J, Spring M, Carroll PV et al (2006) 18Flurodeoxyglucose positron emission tomography in the localization of ectopic ACTH-secreting neuroendocrine tumours. Clin Endocrinol 64:371–374
Basu S, Nair N (2005) 18F-FDG uptake in bilateral adrenal hyperplasia causing Cushing’s syndrome. Eur J Nucl Med Mol Imag 32:384
Markou A, Manning P, Kaya B et al (2005) [18F]fluoro-2-deoxy-D-glucose ([18F]FDG) positron emission tomography imaging of thymic carcinoid tumor presenting with recurrent Cushing’s syndrome. Eur J Endocrinol 152:521–525
Ahmed M, Imaddudin K, Alari A et al (2000) ACTH-producing pituitary cancer: experience at the King Faisal Specialist Hospital & Research Centre. Pituitary 3:105–112
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharp, S.E., Gelfand, M.J. & Absalon, M.J. Altered FDG uptake patterns in pediatric lymphoblastic lymphoma patients receiving induction chemotherapy that includes very high dose corticosteroids. Pediatr Radiol 42, 331–336 (2012). https://doi.org/10.1007/s00247-011-2228-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-011-2228-7