Abstract
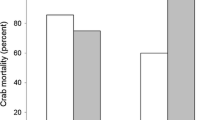
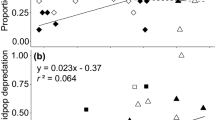
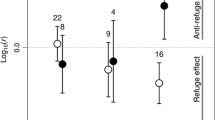
During the last decades, fragmentation has become an important issue in ecological research. Habitat fragmentation operates on spatial scales ranging over several magnitudes from patches to landscapes. We focus on small-scale fragmentation effects relevant to animal foraging decision making that could ultimately generate distribution patterns. In a controlled experimental environment, we tested small-scale fragmentation effects in artificial sea grass on the feeding behaviour of juvenile cod (Gadus morhua). Moreover, we examined the influence of fragmentation on the distribution of one of the juvenile cod’s main prey resources, the grass shrimp (Palaemon elegans), in association with three levels of risk provided by cod (no cod, cod chemical cues and actively foraging cod). Time spent by cod within sea grass was lower in fragmented landscapes, but total shrimp consumption was not affected. Shrimp utilised vegetation to a greater extent in fragmented treatments in combination with active predation. We suggest that shrimp choose between sand and vegetation habitats to minimize risk of predation according to cod habitat-specific foraging capacities, while cod aim to maximize prey-dependent foraging rates, generating a habitat-choice game between predator and prey. Moreover, aggregating behaviour in grass shrimp was only found in treatments with active predation. Hence, we argue that both aggregation and vegetation use are anti-predator defence strategies applied by shrimp. We therefore stress the importance of considering small-scale behavioural mechanisms when evaluating consequences from habitat fragmentation on trophic processes in coastal environments.




Similar content being viewed by others
References
Andren H (1994) Effect of habitat fragmentation on birds and mammals in landscapes with different proportions of suitable habitat: a review. Oikos 71(3):355–366
Baden S, Gullstrom M, Lunden B, Pihl L, Rosenberg R (2003) Vanishing seagrass (Zostera marina, L.) in Swedish coastal waters. Ambio 32(5):374–377
Barnett A, Semmens MS (2012) Sequential movement into coastal habitats and high spatial overlap of predator and prey suggest high predation pressure in protected areas. Oikos 21(6):882–890
Bostrom C, Jackson EL, Simenstad CA (2006) Seagrass landscapes and their effects on associated fauna: a review. Estuar Coast Shelf Sci 68(3–4):383–403. doi:10.1016/j.ecss.2006.01.026
Brown GE (2003) Learning about danger: chemical alarm cues and local risk assessment in prey fishes. Fish Fish 4(3):227–234
Coen LD, Heck KL, Abele LG (1981) Experiments on competition and predation among shrimps of seagrass meadows. Ecology 62(6):1484–1493
Connolly RM, Hindell JS (2006) Review of nekton patterns and ecological processes in seagrass landscapes. Estuar Coast Shelf Sci 68(3–4):433–444. doi:10.1016/j.ecss.2006.01.023
Dupuch A, Dill LM, Magnan P (2009) Testing the effects of resource distribution and inherent habitat riskiness on simultaneous habitat selection by predators and prey. Anim Behav 78(3):705–713. doi:10.1016/J.Anbehav.2009.05.033
Eggleston DB, Etherington LL, Elis WE (1998) Organism response to habitat patchiness: species and habitat-dependent recruitment of decapod crustaceans. J Exp Mar Biol Ecol 223(1):111–132
Evans SR, Finnie M, Manica A (2007) Shoaling preferences in decapod crustacea. Anim Behav 74:1691–1696. doi:10.1016/j.anbehav.2007.03.017
Fahrig L (2003) Effects of habitat fragmentation on biodiversity. In: Futuyma DJ (ed) Annual review of ecology evolution and systematics. Annual review of ecology evolution and systematics, vol 34., pp 487–515
Farina A (1998) Principles and methods in landscape ecology. Hapman and Hall, London
Frank KT, Petrie B, Choi JS, Leggett WC (2005) Trophic cascades in a formerly cod-dominated ecosystem. Science 308(5728):1621–1623. doi:10.1126/Science.1113075
Gorman AM, Gregory RS, Schneider DC (2009) Eelgrass patch size and proximity to the patch edge affect predation risk of recently settled age 0 cod (Gadus). J Exp Mar Biol Ecol 371(1):1–9. doi:10.1016/j.jembe.2008.12.008
Gotceitas V, Fraser S, Brown JA (1995) Habitat use by juvenile Atlantic cod (Gadus morhua) in the presence of an actively foraging and non-foraging predator. Mar Biol (Berlin) 123(3):421–430
Gotceitas V, Fraser S, Brown JA (1997) Use of eelgrass beds (Zostera marina) by juvenile Atlantic cod (Gadus morhua). Can J Fish Aquat Sci 54(6):1306–1319
Holling CS (1959) Some characteristics of simple types of predation and parasitism. Canad Ent Ottawa 91:385–398
Hovel KA, Regan HM (2008) Using an individual-based model to examine the roles of habitat fragmentation and behavior on predator-prey relationships in seagrass landscapes. Landscape Ecol 23(Suppl. 1):75–89. doi:10.1007/s10980-007-9148-9
Hugie DM, Dill LM (1994) Fish and game—a game-theoretic approach to habitat selection by Predators and Prey. J Fish Biol 45:151–169
Irlandi EA (1997) Seagrass patch size and survivorship of an infaunal bivalve. Oikos 78(3):511–518
Irlandi EA, Ambrose WG, Orlando BA (1995) Landscape ecology and the marine environment: how spatial configuration of seagrass habitat influences growth and survival of the Bay Scallop. Oikos 72(3):307–313
Jackson JBC, Kirby MX, Berger WH, Bjorndal KA, Botsford LW, Bourque BJ, Bradbury RH, Cooke R, Erlandson J, Estes JA, Hughes TP, Kidwell S, Lange CB, Lenihan HS, Pandolfi JM, Peterson CH, Steneck RS, Tegner MJ, Warner RR (2001) Historical overfishing and the recent collapse of coastal ecosystems. Science (Washington, DC) 293(5530):629–638
Krause J, Ruxton GD (2002) Living in groups. Oxford University Press, Oxford
Kullmann H, Thunken T, Baldauf SA, Bakker TCM, Frommen JG (2008) Fish odour triggers conspecific attraction behaviour in an aquatic invertebrate. Biol Lett 4(5):458–460. doi:10.1098/rsbl.2008.0246
Larsen TH, Lopera A, Forsyth A (2008) Understanding trait-dependent community disassembly: dung beetles, density functions, and forest fragmentation. Conserv Biol 22:1288–1298
Laurel BJ, Gregory RS, Brown JA (2003) Predator distribution and habitat patch area determine predation rates on age-0 juvenile cod Gadus spp. Mar Ecol Prog Ser 251:245–254
Lima SL (2002) Putting predators back into behavioral predator-prey interactions. Trends Ecol Evol 17(2):70–75
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation—a review and prospectus. Can J Zool Rev Can Zool 68(4):619–640
Micheli F, Peterson CH (1999) Estuarine vegetated habitats as corridors for predator movements. Conserv Biol 13(4):869–881
Moksnes PO, Gullstrom M, Tryman K, Baden S (2008) Trophic cascades in a temperate seagrass community. Oikos 117(5):763–777. doi:10.1111/j.2008.0030-1299.16521.x
Olsson P (2005) Marine field studies in Öresund 2005—Eelgrass. Toxicon, Landskrona (in Swedish)
Persson M, Andersson S, Baden S, Moksnes PO (2008) Trophic role of the omnivorous grass shrimp Palaemon elegans in a Swedish eelgrass system. Mar Ecol Prog Ser 371:203–212. doi:10.3354/meps07674
Persson A, Ljungberg P, Andersson M, Götzman E, Anders Nilsson PA (2012) Foraging performance of juvenile Atlantic cod (Gadus morhua) and profitability of coastal habitats. Mar Ecol Prog Ser. doi:10.3354/meps09705
Pihl L (1982) Food intake of young cod (Gadus morhua) and flounder (Platichthys flesus) in a shallow bay on the Swedish west coast. Neth J Sea Res 15(3–4):419–432
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
R FfSC (2010) R: a language and environment for statistical computing. R Development Core Team. Accessed 4 Nov 2010
Short FT, Wyllie-Echeverria S (1996) Natural and human-induced disturbance of seagrasses. Environ Conserv 23(1):17–27
Valentine J, Duffy JE (2005) The central role of grazing in seagrass ecosystems. In: Larkum AWD, Orth RJ, Duarte CM (eds) Seagrasses: biology, ecology and conservation. Springer, Dordrecht, pp 463–501
Villard MA, Trzcinski MK, Merriam G (1999) Fragmentation effects on forest birds: relative influence of woodland cover and configuration on landscape occupancy. Conserv Biol 13(4):774–783
Waycott M, Duarte CM, Carruthers TJB, Orth RJ, Dennison WC, Olyarnik S, Calladine A, Fourqurean JW, Heck KL, Hughes AR, Kendrick GA, Kenworthy WJ, Short FT, Williams SL (2009) Accelerating loss of seagrasses across the globe threatens coastal ecosystems. P Natl Acad Sci USA 106(30):12377–12381. doi:10.1073/Pnas.0905620106
Wilcove DSMC, Dobson AP (1986) Habitat fragmentation in the temperate zone. In: Soulé M (ed) Conservation biology. Sinauer, Sunderland, pp 237–256
Wisenden BD (2000) Olfactory assessment of predation risk in the aquatic environment. Philos Trans R Soc Lond Ser B Biol Sci 355(1401):1205–1208
Zar JH (1996) Biostatistical analysis. Prentice-Hall, Englewood
Acknowledgments
We thank Alexander Hegg for assistance in the field and Johan Hollander and Melanie Hedgespeth for constructive comments on the manuscript. Financial support was provided from Anna and Edwin Berger Foundation (to PL), Krapperup Foundation (to AP), Oscar & Lili Lamm Memorial Foundation (to AP) and Formas (to AP and PAN). All work was conducted in accordance with ethical laws of Swedish along with permission from Swedish Board of Fisheries.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bulleri.
Rights and permissions
About this article
Cite this article
Ljungberg, P., Hasper, T.B., Nilsson, P.A. et al. Effects of small-scale habitat fragmentation on predator–prey interactions in a temperate sea grass system. Mar Biol 160, 667–675 (2013). https://doi.org/10.1007/s00227-012-2122-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-012-2122-3