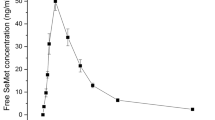
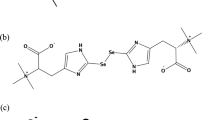
Abstract
We have evaluated the use of 34S-labelled yeast to perform sulphur metabolic tracer experiments in laboratory animals. The proof of principle work included the selection of the culture conditions for the preparation of sulphur labelled yeast, the study of the suitability of this labelled yeast as sulphur source for tracer studies using in vitro gastrointestinal digestion and the administration of the 34S-labelled yeast to laboratory animals to follow the fate and distribution of 34S in the organism. For in vitro gastrointestinal digestion, the combination of sodium dodecyl sulphate-polyacrylamide gel electrophoresis and high-performance liquid chromatography and inductively coupled plasma mass spectrometry (HPLC-ICP-MS) showed that labelled methionine, cysteine and other low molecular weight sulphur-containing biomolecules were the major components in the digested extracts of the labelled yeast. Next, in vivo kinetic experiments were performed in healthy Wistar rats after the oral administration of 34S-labelled yeast. The isotopic composition of total sulphur in tissues, urine and faeces was measured by double-focusing inductively coupled plasma mass spectrometry after microwave digestion. It was observed that measurable isotopic enrichments were detected in all samples. Finally, initial investigations on sulphur isotopic composition of serum and urine samples by HPLC-ICP-MS have been carried out. For serum samples, no conclusive data were obtained. Interestingly, chromatographic analysis of urine samples showed differential isotope enrichment for several sulphur-containing biomolecules.





Similar content being viewed by others
References
Villar-Garea A, Griese M, Imhof A (2007) Biomarker discovery from body fluids using mass spectrometry. J Chromatogr B 849:105–114
Apweiler R, Aslanidis C, Deufel T, Gerstner A, Hansen J, Hochstrasser D et al (2009) Approaching clinical proteomics: current state and future fields of application in fluid proteomics. Clin Chem Lab Med 47:724–744
Silberring J, Ciborowski P (2010) Biomarker discovery and clinical proteomics. TRAC Trends Anal Chem 29:128–140
Srinivas PR, Srivastava S, Hanash S, Wright GL (2001) Proteomics in early detection of cancer. Clin Chem 47:1901–1911
Shau H, Chandler GS, Whitelegge JP, Gornbein JA, Faull KF, Chang HR (2003) Proteomic profiling of cancer biomarkers. Brief Funct Genom Proteomics 2:147–158
Fliser D, Novak J, Thongboonkerd V, Argilés A, Jankowski V, Girolami MA, Jankowski J, Mischak H (2007) Advances in urinary proteome analysis and biomarker discovery. J Am Soc Nephrol 18:1057–1071
Ru QC, Katenhusen RA, Zhu LA, Silberman J, Yang S, Orchard TJ, Brzeski H, Liebman M, Ellsworth DL (2006) Proteomic profiling of human urine using multi-dimensional protein identification technology. J Chromatogr A 1111:166–174
Wittke S, Fliser D, Haubitz M, Bartel S, Krebs R, Hausadel F, Hillmann M, Golovko I, Koester P, Haller H, Kaiser T, Mischak H, Weissinger EM (2003) Determination of peptides and proteins in human urine with capillary electrophoresis–mass spectrometry, a suitable tool for the establishment of new diagnostic markers. J Chromatogr A 1013:173–181
Haubitz M, Wittke S, Weissinger EM, Walden M, Rupprecht HD, Floege J, Haller H, Mischak H (2005) Urine protein patterns can serve as diagnostic tools in patients with IgA nephropathy. Kidney Int 67:2313–2320
Theodorescu D, Wittke S, Ross MM, Walden M, Conaway M, Just I, Mischak H, Frierson HF (2006) Discovery and validation of new protein biomarkers for urothelial cancer: a prospective analysis. Lancet Oncol 7:230–240
Kaiser T, Kamal H, Rank A, Kolb HJ, Holler E, Ganser A, Hertenstein B, Mischak H, Weissinger EM (2004) Proteomics applied to the clinical follow-up of patients after allogeneic hematopoietic stem cell transplantation. Blood 104:340–349
Wind M, Wegener A, Eisenmenger A, Kellner R, Lehmann WD (2003) Sulfur as the key element for quantitative protein analysis by capillary liquid chromatography coupled to element mass spectrometry. Angew Chem Int Ed 42:3425–3427
Prange A, Profrock D (2008) Chemical labels and natural element tags for the quantitative analysis of bio-molecules. J Anal At Spectrom 23:432–459
Rappel C, Schaumlöffel D (2008) The role of sulfur and sulfur isotope dilution analysis in quantitative protein analysis. Anal Bioanal Chem 390:605–615
Anderson JW (1980) Assimilation of inorganic sulfate into cysteine. In: Stumpf PK, Conn EE (eds) The biochemistry of plants, vol 5. Academic, New York, p 203
Porro D, Sauer M, Branduardi P, Mattanovich D (2005) Recombinant protein production in yeasts. Mol Biotechnol 31:245–259
Thomas D, Surdin-Kerjan Y (1997) Metabolism of sulfur amino acids in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 61:503–532
Barnett JA (2003) Beginnings of microbiology and biochemistry: the contribution of yeast research. Microbiology 149:557–567
Giner Martínez-Sierra J, Moreno Sanz F, Herrero Espílez P, Marchante Gayón JM, García Alonso JI (2007) Biosynthesis of sulfur-34 labelled yeast and its characterisation by multicollector-ICP-MS. J Anal At Spectrom 22:1105–1112
Rodríguez-González P, Ruiz Encinar J, García Alonso JI, Sanz-Medel A (2005) Monitoring the degradation and solubilisation of butyltin compounds during in vitro gastrointestinal digestion using “triple spike” isotope dilution GC-ICP-MS. Anal Bioanal Chem 381:380–387
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Rodríguez-González P, García Alonso JI (2010) Recent advances in isotope dilution analysis for elemental speciation. J Anal At Spectrom 25:239–259
Giner Martínez-Sierra J, Moreno Sanz F, Herrero Espílez P, Santamaria-Fernandez R, Marchante Gayón JM, García Alonso JI (2010) Evaluation of different analytical strategies for the quantification of sulfur-containing biomolecules by HPLC-ICP-MS: application to the characterisation of 34S-labelled yeast. J Anal At Spectrom 25:989–997
Rodríguez-González P, Marchante-Gayón JM, García Alonso JI, Sanz-Medel A (2005) Isotope dilution analysis for elemental speciation: a tutorial review. Spectrochim Acta B 60:151–207
Giner Martínez-Sierra J, Santamaria-Fernandez R, Hearn R, Marchante Gayón JM, García Alonso JI (2010) Development of a direct procedure for the measurement of sulfur isotope variability in beers by MC-ICP-MS. J Agric Food Chem 58:4043–4050
Santamaria-Fernandez R, Giner Martínez-Sierra J, Marchante Gayón JM, García Alonso JI, Hearn R (2009) Measurement of longitudinal sulfur isotopic variations by laser ablation MC-ICP-MS in single human hair strands. Anal Bioanal Chem 394:225–233
Tirumalai RS, Chan KC, Prieto DA, Issaq HJ, Conrads TP, Veenstra TD (2003) Characterisation of the low molecular weight human serum proteome. Mol Cell Proteomics 2:1096–1103
Shou M, Conrads TP, Veenstra TD (2005) Proteomic approaches to biomarker detection. Brief Funct Genom Proteomics 4:69–75
Luque-Garcia JL, Neubert TA (2007) Sample preparation for serum/plasma profiling and biomarker identification by mass spectrometry. J Chromatogr A 1153:259–276
Martorella A, Robbins R (2007) Serum peptide profiling: identifying novel cancer biomarkers for early disease detection. Acta Biomed 78:123–128
Linke T, Doraiswamy S, Harrison EH (2007) Rat plasma proteomics: effects of abundant protein depletion on proteomic analysis. J Chromatogr B 849:273–281
Khan A, Packer NH (2006) Simple urinary sample preparation for proteomic analysis. J Proteome Res 5:2824–2838
Magagnotti C, Fermo I, Carletti RM, Ferrari M, Bachi A (2010) Comparison of different depletion strategies for improving resolution of the human urine proteome. Clin Chem Lab Med 48:531–535
Acknowledgments
This work was supported by the Ministry of Science and Innovation, Madrid, Spain (project CTQ2009-12814) and the Education and Science Council of the Principado de Asturias (grant BP07-059). Teresa Fernández and Agustín Brea from the Biotery of the University of Oviedo are gratefully acknowledged for their help and kind suggestions. The authors thank Rafael Peláez for his assistance in the experimental work regarding gels.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Isotope Ratio Measurements: New Developments and Applications with guest editors Klaus G. Heumann and Torsten C. Schmidt.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 628 kb)
Rights and permissions
About this article
Cite this article
Martínez-Sierra, J.G., Sanz, F.M., Espílez, P.H. et al. Sulphur tracer experiments in laboratory animals using 34S-labelled yeast. Anal Bioanal Chem 405, 2889–2899 (2013). https://doi.org/10.1007/s00216-012-6420-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-6420-x