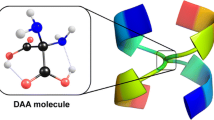
Abstract
The double amino acid (DAA) molecule ((NH2)2C(COOH)2) whose existence was recently proposed is investigated regarding its stability against the unimolecular decarboxylation process in both gas and aqueous phases. The results of the ab initio CCSD(T)/aug-cc-pVDZ//MP2/aug-cc-pVDZ calculations indicate that the spontaneous detachment of carbon dioxide from DAA molecule (regardless of the reaction path chosen) should not be considered as operative despite the fact that the decarboxylation process leading to the most stable products is expected to be thermodynamically slightly favorable (by less than 2 kcal/mol in both gas and aqueous phases). The adequate unimolecular reaction mechanisms of the possible DAA decarboxylation processes are presented and discussed.







Similar content being viewed by others
References
Hu CA, Khalil S, Zhaorigetu S, Liu Z, Tyler M, Wan G, Valle D (2008) Amino Acids 35:665–672
Manna P, Sinha M, Sil PC (2009) Amino Acids 36:417–428
Sako Y, Goto Y, Murakami H, Suga H (2008) ACS Chem Biol 3:241–249
Rogers JM, Suga H (2015) Org Biomol Chem 13:9353–9363
Freza S, Marchaj M, Skurski P (2014) Chem Phys Lett 599:34–37
Freza S (2016) Theor Chem Acc 135:146
Alexandrova AN, Jorgensen WL (2011) J Phys Chem B 115:13624–13632
Li J, Brill TB (2003) J Phys Chem A 107:5993–5997
Li J, Brill TB (2003) J Phys Chem A 107:2667–2673
Li J, Brill TB (2003) J Phys Chem A 107:5987–5992
Snider MJ, Wolfenden R (2000) J Am Chem Soc 122:11507–11508
Boto A, Hernandez R, Suarez E (2000) J Org Chem 65:4930–4937
Steffen LK, Glass RS, Sabahi M, Wilson GS, Schoneich C, Mahling S, Asmus KD (1991) J Am Chem Soc 113:2141–2145
Bonifačić M, Štefanić I, Hug GL, Armstrong DA, Asmus KD (1998) J Am Chem Soc 120:9930–9940
Callahan BP, Wolfenden R (2004) J Am Chem Soc 126:4514–4515
Xiang Z (2013) J Mol Sruct 1049:149–156
Xiang Z (2013) Comput Theor Chem 1006:1–8
Burger E, Tunge J (2006) J Am Chem Soc 128:10002–10003
Boto A, Hernández R, Suárez E (2000) Tetrahedron Lett 41:2899–2902
Boto A, Hernández R, Leon Y, Murguia JR, Rodriguez-Afons A (2004) Tetrahedron Lett 45:6841–6845
Liu D, Hwang CC, Cook PF (2002) Biochemistry 41:12200–12203
Van Poelje PD, Snell EE (1990) Annu Rev Biochem 59:29–59
Kendall RA, Dunning TH Jr, Harrison RJ (1992) J Chem Phys 96:6796–6806
Purvis GD III, Bartlett RJ (1982) J Chem Phys 76:1910–1918
Pople JA, Head-Gordon M, Raghavachari K (1987) J Chem Phys 87:5968–5975
Miertuš S, Scrocco E, Tomasi J (1992) Chem Phys 55:117–129
Miertuš S, Tomasi J (1982) Chem Phys 65:239–245
Cossi M, Barone V, Cammi R, Tomasi J (1996) Chem Phys Lett 255:327–335
Frisch MJ et al (2009) Gaussian09 (Rev.D.01). Gaussian Inc., Wallingford
Acknowledgements
This research was supported by the Polish Ministry of Science and Higher Education Grant No. DS 530-8375-D499-16.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Freza, S. The stability of the double amino acid against decarboxylation in gas and aqueous phases. Theor Chem Acc 136, 7 (2017). https://doi.org/10.1007/s00214-016-2031-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-016-2031-5