Abstract
Background and Objective
Severe neutropenia is the most frequent and important toxicity of 3-weekly paclitaxel and puts patients at substantial risk of infectious complications. It is well known that the time during which paclitaxel plasma concentrations exceed 0.05 μmol/L (TC>0.05) correlates with the extent of neutropenia. This study was initiated to develop a dosing algorithm that would be able to reduce severe neutropenia by targeting an individual paclitaxel TC>0.05 between 26 and 31 hours, and could be validated in a prospective randomized trial by comparing it to conventional dosing of paclitaxel.
Methods
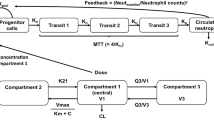
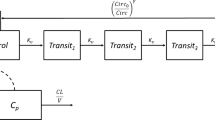
Paclitaxel plasma concentration-time (n = 273) and absolute neutrophil count (ANC) data (152 of the 273 patients) were pooled from two previous studies and submitted to population pharmacokinetic and pharmacodynamic modelling using nonlinear mixed-effects modelling software NONMEM® version VII. To fit the data, we used a previously described 3-compartment model with saturable elimination and distribution, coupled to a semiphysiological model with a linear function to describe the myelotoxic effect of paclitaxel (Epaclitaxel) on circulating neutrophils (neutropenia). Patient age, sex, body surface area (BSA), bilirubin and renal function were tested as potential covariates on the maximum elimination capacity of paclitaxel (VMEL). Limited sampling strategies were tested on the pharmacokinetic model for their accuracy to predict paclitaxel TC>0.05. Subsequently, we proposed a first-cycle dosing algorithm that accounted for BSA, patient age and sex, while later cycles accounted for the previous-cycle paclitaxel TC>0.05 (target: 26 to 31 hours) and ANC nadir to adapt the paclitaxel dose for the next treatment cycle. To test the adequacy of the proposed dosing algorithm, we used extensive data simulations on the final pharmacokinetic/pharma-codynamic model, generating datasets of 1000 patients for six subsequent treatment cycles. Grade 4 neutropenia was tested as a potential endpoint for a prospective clinical trial and simulated for two scenarios, i.e. conventional dosing of paclitaxel 200 mg/m2 every 3 weeks, and personalized, pharmacology-driven dosing as outlined above.
Results
Concentration-time data for paclitaxel were adequately described by the 3-compartment model. Also, individual ANC counts were adequately described by the semiphysiological model using a linear function to describe Epaclitaxel on neutropenia. Patient age, sex, bilirubin and BSA were significant and independent covariates on the elimination of paclitaxel. Paclitaxel VMEL was 16% higher in males than in female patients, and a 10-year increase in age led to a 13% decrease in VMEL. A single paclitaxel plasma concentration 24 hours after the start of infusion was adequate to predict paclitaxel TC>0.05 (root squared mean error [RSME] = +0.5%), and the addition of an end-of-infusion sample did not further improve precision (RSME = −0.6%). Data simulations on the final pharmacokinetic/pharmacodynamic model and using the proposed dosing algorithm resulted in a first-cycle paclitaxel dose ranging from 150 to 185 mg/m2 for women and from 165 to 200 mg/m2 for men. Dose adaptations for cycles two to six ranged from −40% to +30%, with a final median paclitaxel dose of 167 mg/m2 (range 76 to 311 mg/m2). When compared with conventional dosing (paclitaxel 200 mg/m2 every 3 weeks), personalized dosing reduced grade 4 neutropenia in cycle one from 15% to 7%, and further to 4% in cycle 2.
Conclusion
This study proposes a pharmacology-driven dosing algorithm of 3-weekly paclitaxel to reduce the incidence of grade 4 neutropenia. A randomized clinical trial comparing this dosing algorithm with conventional BSA-based dosing of paclitaxel in patients with advanced non-small cell lung cancer is currently ongoing.








Similar content being viewed by others
References
Belani CP, Lee JS, Socinski MA, et al. Randomized phase III trial comparing cisplatin-etoposide to carboplatin-paclitaxel in advanced or metastatic non-small cell lung cancer. Ann Oncol 2005; 16(7): 1069–75
Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 2008; 358(16): 1663–71
Sledge GW, Neuberg D, Bernardo P, et al. Phase III trial of doxorubicin, paclitaxel, and the combination of doxorubicin and paclitaxel as front-line chemotherapy for metastatic breast cancer: an intergroup trial (E1 193). J Clin Oncol 2003; 21(4): 588–92
du Bois A, Luck HJ, Meier W, et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst 2003; 95(17): 1320–9
Gianni L, Kearns CM, Giani A, et al. Nonlinear pharmacokinetics and metabolism of paclitaxel and its pharmacokinetic/pharmacodynamic relationships in humans. J Clin Oncol 1995; 13(1): 180–90
Ohtsu T, Sasaki Y, Tamura T, et al. Clinical pharmacokinetics and pharmacodynamics of paclitaxel: a 3-hour infusion versus a 24-hour infusion. Clin Cancer Res 1995; 1(6): 599–606
Henningsson A, Sparreboom A, Sandstrom M, et al. Population pharmacokinetic modelling of unbound and total plasma concentrations of paclitaxel in cancer patients. Eur J Cancer 2003; 39(8): 1105–14
Karlsson MO, Molnar V, Freijs A, et al. Pharmacokinetic models for the saturable distribution of paclitaxel. Drug Metab Dispos 1999; 27(10): 1220–3
Huizing MT, Vermorken JB, Rosing H, et al. Pharmacokinetics of paclitaxel and three major metabolites in patients with advanced breast carcinoma refractory to anthracycline therapy treated with a 3-hour paclitaxel infusion: a European Cancer Centre (ECC) trial. Ann Oncol 1995; 6(7): 699–704
Joerger M, Huitema AD, Huizing MT, et al. Safety and pharmacology of paclitaxel in patients with impaired liver function: a population pharmacokinetic-pharmacodynamic study. Br J Clin Pharmacol 2007; 64(5): 622–33
Venook AP, Egorin MJ, Rosner GL, et al. Phase I and pharmacokinetic trial of paclitaxel in patients with hepatic dysfunction: Cancer and Leukemia Group B 9264. J Clin Oncol 1998; 16(5): 1811–9
Henningsson A, Karlsson MO, Vigano L, et al. Mechanism-based pharmacokinetic model for paclitaxel. J Clin Oncol 2001; 19(20): 4065–73
Huizing MT, Keung AC, Rosing H, et al. Pharmacokinetics of paclitaxel and metabolites in a randomized comparative study in platinum-pretreated ovarian cancer patients. J Clin Oncol 1993; 11(11): 2127–35
Friberg LE, Henningsson A, Maas H, et al. Model of chemotherapy-induced myelosuppression with parameter consistency across drugs. J Clin Oncol 2002; 20(24): 4713–21
Jennens R, Rischin D, Yuen K, et al. Comparison of neutropenia in a randomized, crossover trial of 3-, 6-, and 24-h infusions of paclitaxel. Gynecol Oncol 2003; 91(1): 190–3
Augusto C, Pietro M, Cinzia M, et al. Peripheral neuropathy due to paclitaxel: study of the temporal relationships between the therapeutic schedule and the clinical quantitative score (QST) and comparison with neurophysiological findings. J Neurooncol 2008; 86(1): 89–99
Blumenschein Jr GR, Khuri FR, von Pawel J, et al. Phase III trial comparing carboplatin, paclitaxel, and bexarotene with carboplatin and paclitaxel in chemotherapy-naive patients with advanced or metastatic non-small-cell lung cancer: SPIRIT II. J Clin Oncol 2008; 26(11): 1879–85
Hensing TA, Peterman AH, Schell MJ, et al. The impact of age on toxicity, response rate, quality of life, and survival in patients with advanced, stage IIIB or IV nonsmall cell lung carcinoma treated with carboplatin and paclitaxel. Cancer 2003; 98(4): 779–88
Herbst RS, Prager D, Hermann R, et al. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol 2005; 23(25): 5892–9
Socinski MA, Schell MJ, Peterman A, et al. Phase III trial comparing a defined duration of therapy versus continuous therapy followed by second-line therapy in advanced-stage IIIB/IV non-small-cell lung cancer. J Clin Oncol 2002; 20(5): 1335–43
Treat JA, Gonin R, Socinski MA, et al. A randomized, phase III multicenter trial of gemcitabine in combination with carboplatin or paclitaxel versus paclitaxel plus carboplatin in patients with advanced or metastatic non-small-cell lung cancer. Ann Oncol 2010; 21(3): 540–7
Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006; 355(24): 2542–50
Ramalingam SS, Dahlberg SE, Langer CJ, et al. Outcomes for elderly, advanced-stage non small-cell lung cancer patients treated with bevacizumab in combination with carboplatin and paclitaxel: analysis of Eastern Cooperative Oncology Group Trial 4599. J Clin Oncol 2008; 26(1): 60–5
Kubota K, Kawahara M, Ogawara M, et al. Vinorelbine plus gemcitabine followed by docetaxel versus carboplatin plus paclitaxel in patients with ad-vanced non-small-cell lung cancer: a randomised, open-label, phase III study. Lancet Oncol 2008; 9(12): 1135–42
Blay JY, Chauvin F, Le Cesne A, et al. Early lymphopenia after cytotoxic chemotherapy as a risk factor for febrile neutropenia. J Clin Oncol 1996; 14(2): 636–43
Silber JH, Fridman M, DiPaola RS, et al. First-cycle blood counts and subsequent neutropenia, dose reduction, or delay in early-stage breast cancer therapy. J Clin Oncol 1998; 16(7): 2392–400
Crawford J, Glaspy JA, Stoller RG, et al. Final results of a placebo-controlled study of filgrastim in small-cell lung cancer: exploration of risk factors for febrile neutropenia. Support Cancer Ther 2005; 3(1): 36–46
Joerger M, Huitema AD, Richel DJ, et al. Population pharmacokinetics and pharmacodynamics of paclitaxel and carboplatin in ovarian cancer patients: a study by the European organization for research and treatment of cancer-pharmacology and molecular mechanisms group and new drug development group. Clin Cancer Res 2007; 13(21): 6410–8
Miller AA, Rosner GL, Egorin MJ, et al. Prospective evaluation of body surface area as a determinant of paclitaxel pharmacokinetics and phar-macodynamics in women with solid tumors: Cancer and Leukemia Group B study 9763. Clin Cancer Res 2004; 10(24): 8325–31
Mould DR, Fleming GF, Darcy KM, et al. Population analysis of a 24-h paclitaxel infusion in advanced endometrial cancer: a gynaecological oncol-ogy group study. Br J Clin Pharmacol 2006; 62(1): 56–70
Huizing MT, Giaccone G, van Warmerdam LJ, et al. Pharmacokinetics of paclitaxel and carboplatin in a dose-escalating and dose-sequencing study in patients with non-small-cell lung cancer: the European Cancer Centre. J Clin Oncol 1997; 15(1): 317–29
Jiko M, Yano I, Sato E, et al. Pharmacokinetics and pharmacodynamics of paclitaxel with carboplatin or gemcitabine, and effects of CYP3A5 and MDR1 polymorphisms in patients with urogenital cancers. Int J Clin Oncol 2007; 12(4): 284–90
Kobayashi M, Oba K, Sakamoto J, et al. Pharmacokinetic study of weekly administration dose of paclitaxel in patients with advanced or recurrent gastric cancer in Japan. Gastric Cancer 2007; 10(1): 52–7
Mielke S, Sparreboom A, Steinberg SM, et al. Association of paclitaxel pharmacokinetics with the development of peripheral neuropathy in patients with advanced cancer. Clin Cancer Res 2005; 11(13): 4843–50
Nakajima M, Fujiki Y, Kyo S, et al. Pharmacokinetics of paclitaxel in ovarian cancer patients and genetic polymorphisms of CYP2C8, CYP3A4, and MDR1. J Clin Pharmacol 2005; 45(6): 674–82
de Jonge ME, van den Bongard HJ, Huitema AD, et al. Bayesian pharmaco-kinetically guided dosing of paclitaxel in patients with non-small cell lung cancer. Clin Cancer Res 2004; 10(7): 2237–44
EU Clinical Trials Register. An open-label, randomized, parallel group study of patients treated with paclitaxel with standard dosing versus pharm-acokinetic guided dose adjustment in patients with advanced NSCLC. EudraCT number 2010-023688-16 [online]. Available from URL: https://www.clinicaltrialsregister.eu/ctr-search/trial/2010-023688-16/DE [Accessed 2012 Jun 13]
Joerger M, Huitema AD, van den Bongard DH, et al. Quantitative effect of gender, age, liver function, and body size on the population pharmacokinetics of paclitaxel in patients with solid tumors. Clin Cancer Res 2006; 12 (7 Pt 1): 2150–7
Huizing MT, Sparreboom A, Rosing H, et al. Quantification of paclitaxel metabolites in human plasma by high-performance liquid chromatography. J Chromatogr B Biomed Appl 1995; 674(2): 261–8
Beal S, Sheiner LB, Boeckmann A, et al. NONMEM user’s guides 1989–2009. Ellicott City (MD): Icon Development Solutions, 2009
Minami H, Sasaki Y, Watanabe T, et al. Pharmacodynamic modeling of the entire time course of leukopenia after a 3-hour infusion of paclitaxel. Jpn J Cancer Res 2001; 92(2): 231–8
Bulitta JB, Zhao P, Arnold RD, et al. Multiple-pool cell lifespan models for neutropenia to assess the population pharmacodynamics of unbound paclitaxel from two formulations in cancer patients. Cancer Chemother Pharmacol 2009; 63(6): 1035–48
Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361(10): 947–57
Ranson M, Davidson N, Nicolson M, et al. Randomized trial of paclitaxel plus supportive care versus supportive care for patients with advanced non-small-cell lung cancer. J Natl Cancer Inst 2000; 92(13): 1074–80
Gomez H, Hidalgo M, Casanova L, et al. Risk factors for treatment-related death in elderly patients with aggressive non-Hodgkin’s lymphoma: results of a multivariate analysis. J Clin Oncol 1998; 16(6): 2065–9
Caggiano V, Stolshek BS, Delgado DJ, et al. First and all cycle febrile neutropenia hospitalizations (FNH) and costs in intermediate grade non-Hodgkins lymphoma (IGL) patients on standard-dose CHOP therapy [abstract no. 1810]. Blood 2001; 98: 431a
Meza L, Baselga J, Holmes FA, et al. Incidence of febrile neutropenia (FN) is directly related to duration of severe neutropenia (DSN) after myelosuppressive chemotherapy [abstract no. 2840]. Proc Am Soc Clin Oncol 2002; 21: 255b
Mayordomo JI, Lopez A, Vinolas N, et al. Retrospective cost analysis of management of febrile neutropenia in cancer patients in Spain. Curr Med Res Opin 2009; 25(10): 2533–42
Woo MH, Relling MV, Sonnichsen DS, et al. Phase I targeted systemic exposure study of paclitaxel in children with refractory acute leukemias. Clin Cancer Res 1999; 5(3): 543–9
van der Bol JM, Mathijssen RH, Creemers GJ, et al. A CYP3A4 phenotype-based dosing algorithm for individualized treatment of irinotecan. Clin Cancer Res 2010; 16(2): 736–42
Mielke S, Sparreboom A, Behringer D, et al. Paclitaxel pharmacokinetics and response to chemotherapy in patients with advanced cancer treated with a weekly regimen. Anticancer Res 2005; 25(6C): 4423–7
Gonzalez-Angulo AM, Hortobagyi GN. Optimal schedule of paclitaxel: weekly is better. J Clin Oncol 2008; 26(10): 1585–7
Seidman AD, Berry D, Cirrincione C, et al. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol 2008; 26(10): 1642–9
Belani CP, Ramalingam S, Perry MC, et al. Randomized, phase III study of weekly paclitaxel in combination with carboplatin versus standard every-3-weeks administration of carboplatin and paclitaxel for patients with previously untreated advanced non-small-cell lung cancer. J Clin Oncol 2008; 26(3): 468–73
Acknowledgements
Dr Feiss is an employee of Saladox Biomedical, Inc. No sources of funding were used to conduct this study or prepare this manuscript. The authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Joerger, M., Kraff, S., Huitema, A.D.R. et al. Evaluation of a Pharmacology-Driven Dosing Algorithm of 3-Weekly Paclitaxel Using Therapeutic Drug Monitoring. Clin Pharmacokinet 51, 607–617 (2012). https://doi.org/10.1007/BF03261934
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03261934