Abstract
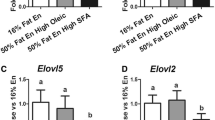
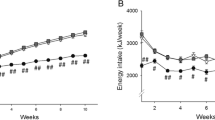
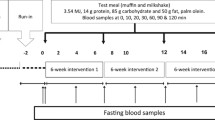
To test the possibility that dietary palmitic acid (16∶0) may be lithogenic, different fats were blended to exchange 18∶1 in olive oil with either 16∶0 in palm stearin, 12∶0+14∶0 in coconut oil, or 14∶0+16∶0 in butterfat. Dietary 18∶2 was held constant at 1.2% energy (en) (with extra safflower oil as needed) in these four purified diets containing low fat (11% of total energy) and 0.4% cholesterol. A fifth, high-fat diet provided 40% of the total energy as the 16∶0-rich blend. All hamsters fed the low-fat, 16∶0-rich blend for six weeks developed cholesterol gallstones (8/8). Although the gallstone incidence was lower for the 12∶0+14∶0-rich diet (5/8), the severity of stone formation in affected hamsters was equal to that in the low-fat, 16∶0-rich group. Mucin accumulation in gallbladder bile was often associated with cholesterol gallstones in diets containing 16∶0, but was minimal in 18∶1-rich and 12∶0+14∶0-rich groups. Neither the lithogenic index (all>1.0), plasma lipids, nor liver cholesterol was a selective predictor of stone formation. The high-fat, 16∶0-rich diet actually decreased cholesterol stone incidence (3/8) and severity, but yielded a high incidence of pigment stones (5/8). Thus, saturated fat and 16∶0per se were not responsible for the exaggerated lithogenesis. Because the antilithogenic 18∶1-rich diet also normalized the 18∶2 intake (1.2% en) relative to previous butter diets (0.3% en), the potential importance of essential fatty acids (EFA) deficiency in the model was tested in a second study by feeding graded amounts of 18∶2 (0.3, 0.6, 0.9, and 1.2% en) as safflower oil in four low-fat, butter-rich diets (11% en as fat) without alleviating gallstone incidence or severity. These studies indicate that substitution of 18∶1 for saturated fatty acids in low-fat diets reduces gallstone formation without affecting the lithogenic index. Furthermore, intake of 18∶2 at or below the EFA requirement does not appear to be a major factor in this model.
Similar content being viewed by others
Abbreviations
- ACAT:
-
acyl cholesterol acyl transferase
- ANOVA:
-
analysis of variance
- apo B:
-
apolipoprotein B
- apo E:
-
apolipoprotein E
- CE:
-
cholesteryl ester
- EFA:
-
essential fatty acid
- en:
-
energy
- HDL:
-
high-density lipoprotein
- HI:
-
hydrophobicity index
- HPLC:
-
high-performance liquid chromatography
- LDL:
-
low-density lipoprotein
- LDLi:
-
low density lipoprotein receptor
- LI:
-
lithogenic index
- PUFA:
-
polyunsaturated fatty acids
- TC:
-
total cholesterol
References
Hayes, K.C., Livingston, A., and Trautwein, E.A. (1992) “Dietary Impact on Biliary Lipids and Gallstones,”Annu. Rev. Nutr. 12, 299–326.
Liepa, G., Gorman, M.A., and Duffy, A.M. (1988) “The Use of Animals in Studying the Effects of Diet on Gallstone Formation,” inComparative Animal Nutrition (Beynen, A.C., and West, C.E., eds.) Vol. 6, pp. 149–173, S. Karger, Basel, Switzerland.
Tanimura, H. (1965) “Experimental Studies on the Etiology of Cholelithiasis,”Arch. Jpn. Chir. 34, 1160–1179.
Wheeler, H.O. (1973) “Biliary Excretion of Bile Acids, Lecithin, and Cholesterol in Hamsters with Gallstones,”Gastroenterol. 65, 92–103.
Bennion, L.J., and Grundy, S.M. (1978) “Risk Factors for the Development of Cholelithiasis in Man,”New Engl. J. Med. 299, 1221–1227.
Maclure, K.M., Hayes, K.C., Colditz, G.A., Stampfer, M.J., and Willett, W.C. (1990) “Dietary Predictors of Symptom-associated Gallstones in Middle-aged Women,”Am. J. Clin. Nutr. 52, 916–922.
Trautwein, E.A., Liang, J., and Hayes, K.C. (1993) “Cholesterol Gallstone Induction in Hamsters Reflects Strain Differences in Plasma Lipoproteins and Bile Acid Profiles,”Lipids 28, 305–312.
Cohen, B.I., Mosbach, E.H., Ayyad, N., Miki, S., and McSherry, C.K. (1992) “Dietary Fat and Fatty Acids Modulate Cholesterol Cholelithiasis in the Hamster,”Lipids 27, 526–532.
Shioda, R. (1965) “Experimental Studies or Gallstone Formation,”Arch. Jpn. Chir. 34, 571–585.
Dam, H. (1971) “Determinants of Cholesterol Cholelithiasis in Man and Animals,”Am. J. Med. 51, 596–613.
Weingard, K.W., and Daggy, B.P. (1990) “Quantification of HDL Cholesterol in Plasma from Hamsters by Differential Precipitation,”Clin. Chem. 36, 575.
Hayes, K.C., Stephan, Z.F., Pronczuk, A., Lindsey, S., and Verdon, C. (1989) “Lactose Protects Against Estrogen-induced Pigment Gallstones in Hamsters Fed Nutritionally Adequate Purified Diets,”J. Nutr. 119, 1726–1736.
Folch, J., Lees, M., and Stanley, G.H.S. (1957) “A Simple Method for the Isolation and Purification of Total Lipids from Animal Tissue,”J. Biol. Chem. 226, 497–509.
Rossi, S.S., Converse, J.L., and Hofmann, A.F. (1987) “High-Pressure Liquid Chromatographic Analysis of Conjugated Bile Acids in Human Bile: Simultaneous Resolution of Sulfated and Unsulfated Lithocholyl Amidates and The Commonly Conjugated Bile Acids,”J. Lipid Res. 28, 589–595.
Trautwein, E.A., Liang, J., and Hayes, K.C. (1993) “Plasma Lipoproteins, Biliary Lipids and Bile Acid Profile Differ in Various Strains of Syrian Hamsters,”Comp. Biochem. Physiol. 104A, 829–835.
Carey, M.C., and Small, D.M. (1978) “The Physical Chemistry of Cholesterol Solubility in Bile: Relationship to Gallstone Formation and Dissolution in Man,”J. Clin. Invest. 61, 998–1026.
Heuman, D.M. (1989) “Quantitative Estimation of the Hydrophilic-Hydrophobic Balance of Mixed Bile Salt Solutions,”J. Lipid Res. 30, 719–730.
Kim, J.C., and Chung, T.H. (1984) “Direct Determination of the Free Cholesterol and Individual Cholesteryl Esters in Serum by HPLC,”Korean J. Biochem. 16, 69–77.
Suckling, K.E., Benson, G.M., Bond, B., Gee, A., Glen, A., Haynes, C., and Jackson, B. (1991) “Cholesterol Lowering and Bile Acid Excretion in the Hamster with Cholestyramine Treatment,”Atherosclerosis 89, 183–190.
Cohen, B.I., Matoba, N., Mosbach, E.H., and McSherry, C. (1989) “Dietary Induction of Cholesterol Gallstones in Hamsters from Three Different Sources,”Lipids 24, 151–156.
Hayes, K.C., Khosla, P., Kaiser, A., Yeghiazarians, V., and Pronczuk, A. (1992) “Dietary Fat and Cholesterol Modulate the Plasma Lipoprotein Distribution and Production of Pigment or Cholesterol Gallstones in Hamsters,”J. Nutr. 122, 374–384.
Ayyad, N., Cohen, B.I., Mosbach, E.H., and Miki, S. (1992) “Palmitic Acid Enhances Cholesterol Gallstone Incidence in Sasco Hamsters Fed Cholesterol Enriched Diets,”Lipids 27, 993–998.
Carey, M.C. (1989) “Formation of Cholesterol Gallstones: the New Paradigms,” inTrends in Bile Acid Research (Paumgartner, G., Stiehl, A., and Gerok, W., eds.), pp. 259–281, Kluwer Academic Publishers, Dordricht/Boston/London.
Spady, D.K., Woollett, L.A., and Dietschy, J.M. (1993) “Regulation of Plasma LDL-Cholesterol Levels by Dietary Cholesterol and Fatty Acids,”Ann. Rev. Nutr. 13, 355–381.
Ginsberg, R.L., Duane, W.C., and Flock, E.V. (1977) “Hepatic 3-Hydroxy-3-Methylglutaryl CoA Reductase Activity in Hamsters on a Lithogenic Diet,”J. Lab. Clin. Med. 89, 928–936.
Ochoa, B., Gee, A., Jackson, B., and Suckling, K.E. (1990) “Regulation of Cholesteryl Ester Metabolism in the Hamster Liver,”Biochim. Biophys. Acta 1044, 133–138.
Daumerie, C.M., Woollett, L.A., and Dietschy, J.M. (1992) “Fatty Acids Regulate Hepatic Low Density Lipoprotein Receptor Activity Through Redistribution of Intracellular Cholesterol Pools,”Proc. Natl. Acad. Sci. USA 89, 10797–10801.
Woollett, L.A., Spady, D.K., and Dietschy, J.M. (1992) “Saturated and Unsaturated Fatty Acids Independently Regulate Low Density Lipoprotein Receptor Activity and Production Rate,”J. Lipid Res. 33, 77–88.
Spady, D.K., and Dietschy, J.M. (1988) “Interaction of Dietary Cholesterol and Triglycerides in the Regulation of Hepatic LDL Transport in the Hamster,”J. Clin. Invest. 81, 300–309.
Turley, S.D., Spady, D.K., and Dietschy, J.M. (1983) “Alteration of the Degree of Biliary Cholesterol Saturation in the Hamster and Rat by Manipulation of the Pools of Preformed and Newly Synthesized Cholesterol,”Gastroenterol. 84, 253–264.
Spady, D.K., Turley, S.D., and Dietschy, J.M. (1983) “Dissociation of Hepatic Cholesterol Synthesis from Hepatic LDL Uptake Ad Biliary Cholesterol Saturation in Female and Male Hamsters of Different Ages,”Biochim. Biophys. Acta 753, 381–392.
Gustafsson, B.F., Einarsson, K., and Gustafsson, J.A. (1975) “Influence of Cholesterol Feeding on Liver Microsomal Metabolism of Steroids and Bile Acids in Conventional and Germ-free Rats,”J. Biol. Chem. 250, 8496–8502.
Trautwein, E.A., Siddiqui, A., and Hayes, K.C. (1993) “Modelling Plasma Lipoprotein-bile Lipid Relationships: Differential Impact of Psyllium and Cholestyramine in Hamsters Fed a Lithogenic Diet,”Metabolism 42, 1531–1540.
Singhal, A.K., Finver-Sadowsky, J., McSherry, C.K., and Mosbach, E.H. (1983) “Effect of Cholesterol and Bile Acids on the Regulation of Cholesterol Metabolism in Hamster”,Biochim. Biophys. Acta 752, 214–222.
Kuroki, S., Muramoto, S., Kuramoto, T., and Hoshita, T. (1983) “Effect of Feeding Cholesterol and Sitosterol on Hepatic Steroid 12 α-Hydroxylase Activity in Female Hamsters,”J. Pharm. Dyn. 6, 551–557.
Author information
Authors and Affiliations
About this article
Cite this article
Jonnalagadda, S.S., Trautwein, E.A. & Hayes, K.C. Dietary fats rich in saturated fatty acids (12∶0, 14∶0, and 16∶0) enhance gallstone formation relative to monounsaturated fat (18∶1) in cholesterol-fed hamsters. Lipids 30, 415–424 (1995). https://doi.org/10.1007/BF02536299
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02536299