Conclusions
-
1.
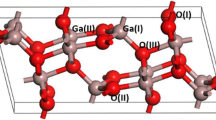
Using seven independent methods it was found that the concentration of the active centers on the surface ofγ-Al2O3, which are responsible for the dehydration, is (9±1)×1017 centers/m2. The catalytically active centers are identical with adsorption centers.
-
2.
A method of calculating the stochiometry, concentration, and thermodynamics of the surface association reactions has been developed.
-
3.
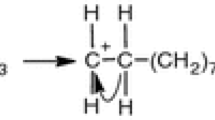
The first reaction step proceeds according to the associative bimolecular substitution mechanism, with the formation of an associative intermediate compound. The association enthalpy and entropy have been calculated.
Similar content being viewed by others
Literature cited
A. Grigor'ev, Zh. Russk. Fiz.-Khim. Obshch.,33, 173 (1901).
V. Ipat'ev, Zh. Russk. Fiz.-Khim. Obshch.,34, 1057 (1902);34, 1098 (1902);35, 449 (1903).
V. N. Ipat'ev, Catalytic Reactions at High Temperatures and Pressures [in Russian], Izd. Akad. Nauk SSSR, Moscow, Leningrad (1936).
P. Sabatier, Le Catalyse en Chimie Organique, C. Béranger, Paris, Liege (1920).
J. B. Senderens, Bull. Soc. Chim. France,4, No. 1, 692 (1907).
T. V. Antipina and A. V. Frost, Usp. Khim.,19, 342 (1950); Dokl. Akad. Nauk SSSR,71, 3737 (1949); A. A. Balandin, Vestn. Mosk. Universit., Ser. “Khimiya”,4, 137 (1957); G. M. Panchenkov, Zh. Fiz. Khim.,22, 209 (1949); K. V. Topchieva and K. Yun-Pin, Zh. Fiz. Khim., 29, 2076 (1955); 29, 1854 (1955).
G. Pains and Dzh. Manassen, Catalysis. Stereochemistry and Mechanisms of Organic Reactions [Russian translation], IL, Moscow (1968), p. 108; H. Knözinger, Angew. Chem.,80, 778 (1968); H. Knözinger and P. Kömie, J. Catal.,3, 559 (1964); H. Knözinger and A. Schglila, J. Catal.,17, 252 (1970).
H. Pines and C. N. Pillai, J. Amer. Chem. Soc.,83, 3270 (1961).
V. V. Sadovnikov and A. M. Gefter, Conference on Mechanism of Heterogeneous Catalytic Reactions [in Russian], Izd. Inst. Khim. Fiz., Akad. Nauk SSSR, Moscow (1974).
O. V. Krylov and E. A. Fokina, Kinet. Katal.,1, 542 (1960); G. V. Isagulyants, A. A. Balandin, and E. I. Popov, Dokl. Akad. Nauk SSSR,139, 139 (1961).
S. Z. Roginskii, M. I. Yanovskii, and A. D. Berman, Principles of Application of Chromatography in Catalysis [in Russian], Izd. Nauka, Moscow (1972).
V. V. Sadovnikov, Dokl. Akad. Nauk SSSR,217, 872 (1974).
J. B. Pari, J. Phys. Chem.,69, 211 (1965);69, 220 (1965).
K. Dorffel, Statistik in der Analytischen Chemie [Russian translation], Deutscher Verlag fur Grundstoffindustrie, Leipzig (1966).
D. Stall, É. Bestram, and G. Zinke, Chemical Thermodynamics of Organic Compounds [Russian translation], Mir (1971).
G. C. Pimentel and A. L. McClellan, The Hydrogen Bond, Reinhold Pub. Corp., New York (1960).
V. I. Vedeneev, L. V. Gurvich, V. N. Kondrat'ev, V. A. Medvedev, and E. L. Frankevich, Cleavage Energies of the Chemical Bonds [in Russian], Izd. Akad. Nauk SSSR, Moscow (1962).
Author information
Authors and Affiliations
Additional information
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 6, pp. 1220–1227, 1976.
The authors thank O. V. Krylova and L. V. Margolis for their assistance in analyzing the results.
Rights and permissions
About this article
Cite this article
Sadovnikov, V.V., Gefter, A.M. Energetic aspects of the first step in the dehydration reaction of i-C3H7OH ON γ-Al2O3 . Russ Chem Bull 25, 1184–1189 (1976). https://doi.org/10.1007/BF00928047
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00928047