Summary
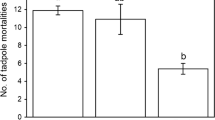
Damselfly larvae may autotomize and regenerate any of their 3 caudal lamellae. At least one missing or regenerating lamella was evident in 50.1% of field collected Ischnura posita larvae. Lamellae loss during molting is very infrequent (1 out of 117 recorded molts). Laboratory trials indicate that conspecifics remove lamellae and that this process is density dependent. The percentage of larvae losing lamellae during 24 h trials ranged from 73.5 at the highest density tested to 17.3 at the lowest density. I. posita larvae are cannibalistic. The presence of lamellae reduces an individual's chance of being cannibalized. More than twice as many final instar lamellae-less larvae were cannibalized during 24 h trials than analogous individuals having 3 lamellae at experimental initiation. Costs are also associated with lamellae autotomy. 1) Although individuals without lamellae can swim they are more reluctant to release from a wooden stalk and swim when threatened (9% release) than are larvae with lamellae (29% release). Since swimming is part of their repertoire of anti-predator behaviors this behavioral shift should be detrimental. 2) Caudal lamellae function in O2 uptake. Trials were conducted with larvae having and not having lamellae in an experimental horizontal oxygen gradient system. Relative to larvae without lamellae, those with lamellae preferred deeper depths at PO2 values greater than 70 torr. Many lamellae-less larvae distributed themselves at the water surface throughout the range of PO2 values tested. Differential depth distribution between larvae with and without lamellae is highly significant (P < 0.01).
Similar content being viewed by others
References
Baker RL, Dixon SM (1986) Wounding as an index of aggressive interactions in larval Zygoptera (Odonata). Can J Zool 64:893–897
Benke AC (1978) Interactions among coexisting predators: A field experiment with dragonfly larvae. J Anim Ecol 47:335–350
Burggren WW (1982) ‘Air gulping’ improves blood oxygen transport during aquatic hypoxia in the goldfish, Carassius auratus. Phys Zool 55:327–334
Byers CF (1930) A contribution to the knowledge of Florida Odonata. Florida Pub Biol Sci 1:1–327
Child CM, Young AN (1903) Regeneration of the appendages in nymphs of the Agrionidae. Archiv fur Entwickelungsmechanik der Organismen 15:543–602, Plates XX–XXII
Chutter FM (1961) Certain aspects of the morphology and ecology of the nymphs of several species of Pseudagrion Selys (Odonata). Arch Hydrobiol 57:430–463
Congdon JD, Vitt LJ, King WW (1974) Geckos: Adaptive significance and energetics of tail autotomy. Science 184:1379–1380
Corbet PS (1950) Missing caudal lamellae in Odonata naidas. Entomologist 83:18
Corbet PS (1957) The life-history of the Emperor Dragonfly Anax imperator Leach (Odonata: Aeshnidae). J Anim Ecol 26:1–69
Corbet PS (1962) A Biology of Dragonflies. E.W. Classey Limited, Faringdon, United Kingdom, 247 p
Crowder LB, Cooper WE (1982) Habitat structural complexity and the interaction between bluegills and their prey. Ecology 63:1802–1813
Crowley PH (1984) Evolutionarily stable strategies for larval damselflies. In: Lecture Notes in Biomathematics: Mathematical Ecology vol 54 edited by S.Levin and T.Hallam, pages 55–74 Springer, Berlin Heidelberg New York Tokyo
Crowley PH, Nisbet RM, Gurney WSC, Lawton JH (1987) Population regulation in animals with complex life-histories: formulation and analysis of a damselfly model. Adv Ecol Res 17:1–59
Dehadrai PV, Tripathi SD (1976) Environment and ecology of freshwater air-breathing teleosts. In: Respiration of Amphibious Vertebrates, G.M. Hughes (editor), pages 39–72. Academic Press, New York, New York
Dejours P (1976) Water versus air as the respiratory medium. In: Respiration of Amphibious Vertebrates, G.M. Hughes (editor), pages 1–15. Academic Press, New York, New York
Eriksen CH (1984), The physiological ecology of larval Lestes disjunctus Selys (Zygoptera: Odonata). Freshw Inv Biol 3:105–117
Eriksen CH (1986) Respiratory roles of caudal lamellae (gills) in a lestid damselfly (Odonata: Zygoptera). JN Amer Benth Soc 5:16–27
Fischer Z (1961a) Cannibalism among the larvae of the dragonfly Lestes nympha Seyls. Ekologia Polska Seria B 7:33–39
Fischer Z (1961b) Some data on the Odonata larvae of small pools. Internationale Reyue der gesamten Hydrobiologie 46:269–275
Harnisch O (1958) Untersuchungen an den analkiemen der larve von Agrion. Biologisches Zentralblatt 77:300–310
Henrikson BI (1988) The absence of antipredator behaviour in the larvae of Leucorrhinia dubia (Odonata) and the consequences for their distribution. Oikos 51:179–183
Johnson C (1972) The Damselflies (Zygoptera) of Texas. Bull Flor State Mus Biol Sci 16:55–128
Johnson DM, Akre BG, Crowley PH (1975) Modeling arthropod predation: wasteful killing by damselfly naiads. Ecology 56:1081–1093
Kramer DL (1982) The evolutionary ecology of respiratory mode in fishes: an analysis based on the costs of breathing. Env Biol Fishes 9:145–158
Kramer DL, Lindsey CC, Mooddie GEE, Stevens ED (1978) The fishes and the aquatic environment of the central Amazon basin, with particular reference to respiratory patterns. Can J Zool 56:717–729
Lawton JH (1970) Feeding and food assimilation in larvae of the damselfly Pyrrhosoma nymphula (Sulzer) (Odonata: Zygoptera). J Anim Ecol 39:669–689
Macan TT (1964) The Odonata of a moorland fishpond. Internationale Revue der Gesamten Hydrobiologie 49:325–360
MacNeill N (1960) A study of the caudal gills of dragonfly larvae of the sub-order Zygoptera. Proc Royal Irish Acad 61(B):115–140
McPeek MA, Crowley PH (1987) The effects of density and relative size on the aggressive behaviour, movement and feeding of damselfly larvae (Odonata: Coenagrionidae). Anim Beh 35:1051–1061
Merrill RJ, Johnson DM (1984) Dietary niche overlap and mutual predation among coexisting larval Anisoptera. Odonatologica 13:387–406
Morin PJ (1984a) The impact of fish exclusion on the abundance and species composition of larval odonates: results of short term experiments in a North Carolina farm pond. Ecology 65:53–60
Morin PJ (1984b) Odonate guild composition: experiments with colonization history and fish predation. Ecology 65:1866–1873
Pearlstone PSM (1973) The food of damselfly larvae in Marion Lake, British Columbia. Syesis 6:33–39
Pennak RW, McColl CM (1944) An experimental study of oxygen absorption in some damselfly naiads. J Cell Comp Phys 23:1–10
Pritchard G (1964) The prey of dragonfly larvae in ponds in northern Alberta. Can J Zool 42:785–800
Robinson JV, Hayworth DA, Harvey MB (1991) The effect of caudal lamellae loss on swimming speed of the damselfly, Argia moesta (Hagen) (Odonata: Coenagrionidae). Am Midl Nat
Robinson JV, Wellborn GA (1987) Mutual predation in assembled communities of odonate species. Ecology 68:921–927
Schmidt E (1919) Uber das schwimmen der libellenlarven. Zoologische Anzeiger 50:235–237
Sokal RR, Rohlf FJ (1981) Biometry (second edition). W.H. Freeman, San Francisco
Thompson DJ (1978) The natural prey of damselfly larvae, Ischnura elegans (Odonata: Zygoptera). Freshw Biol 8:377–384
Tillyard RJ (1917) The Biology of Dragonflies (Odonata: Paraneuroptera). Cambridge University Press
Walker EM (1953) The Odonata of Canada and Alaska, Volume 1. University of Toronto Press, Toronto, Canada. xi 292 p
Wellborn GA, Robinson JV (1987) Microhabitat selection as an antipredatory strategy in the aquatic insect, Pachydiplax longipennis Burmeister (Odonata: Libellulidae). Oecologia 71:185–189
Williams F (1936) Biological studies in Hawaiian water-loving insects. Haw Ent Soc Proc 9:235–349
Wilson CB (1917) Dragonflies and damselflies in relation to pondfish culture, with a list of those found near Fairport, Iowa. Bull Bur Fish 36:181–264
Wissinger SA (1988) Effects of food availability on larval development and inter-instar predation among larvae of Libellula lydia and Libellula luctuosa (Odonata: Anisoptera). Can J Zool 66:543–549
Zahner R (1959) Uber die bindung der mitelleuropaischen Calopteryx-Arten (Odonata, Zygoptera) an den lebensraum des stromenden wassers. I. Der anteil der larven an der biotopbindung. Internationale Revue der Gesamten Hydrobiologie 44:51–130
Zaret TM (1980) Predation and Freshwater Communities. Yale University Press, New Haven
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Robinson, J.V., Shaffer, L.R., Hagemier, D.D. et al. The ecological role of caudal lamellae loss in the larval damselfly, Ischnura posita (Hagen) (Odonata: Zygoptera). Oecologia 87, 1–7 (1991). https://doi.org/10.1007/BF00323773
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00323773