Abstract
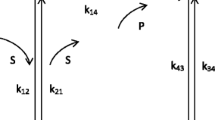
In the present work we have modelled and optimized the reaction mechanism of the triose phosphate isomerase (TIM) enzyme (E.C. 5.3.1.1). For this purpose we have used an approach that combines the S-system representation within the power law formalism and linear programming techniques. By this means we have explored those rate constants whose alterations are likely to improve the catalytic efficiency of the enzyme and investigated the available room for optimization in different metabolic conditions. The role and plausibility of the different types of mutations on the evolution of this enzyme have also been considered.
Steady state sensitivity analysis was carried out and a new set of aggregated logarithmic gains was defined in order to quantify the responses of the system to changes in groups of rate constants that could be explained in terms of mutations affecting the catalytic properties of the enzyme. Evaluation of these logarithmic gains at different levels of saturation and disequilibrium ratios enabled us to reach conclusions about the meaning and role of the diffusion limitation terms.
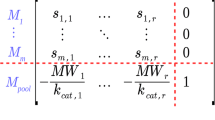
The catalytic efficiency of the monoenzymatic system was optimized through changes in the kinetic rate constants within different sets of restrictions ranging from thermodynamic or kinetic to evolutionary ones. Results showed that, at very different conditions, there is still room for improvement in the TIM enzyme. Thus, in a wide range of metabolically significant values of the disequilibrium ratio there is a minimal variation in the optimal profile that yields 2.1 times the velocity of the basal states. Though most of this increase is accounted for by the increase of the second order constants (that could have already reached a theoretical maximum) significant increases (10%) in catalytic efficiencies are obtained by changes of the internal steps only. Besides these new findings our optimization approach has been able to reproduce results obtained with other approaches.
Similar content being viewed by others
References
Albery, W. J. and J. R. Knowles (1976). Evolution of enzyme function and development of catalytic efficiency. Biochemistry 15, 5631–5640.
Alvarez-Vazquez, F. and N. V. Torres (2000). Metabolism of citric acid production by Aspergillus niger: model definition, steady-state analysis and constrained optimization of citric acid production rate. Biotechnol. Bioeng. 70, 82–108.
Blacklow, S. C., R. T. Raines, W. A. Lim, P. D. Zamore and J. R. Knowles (1988). Triosephosphate isomerase catalysis is diffusion controlled. Biochemistry 27, 1158–1167.
Brown, G. C. (1991). Total cell protein concentration as an evolutionary constraint on the metabolic control distribution in cells. J. Theor. Biol. 153, 195–203.
Brown, G. C. and C. Cooper (1993). Control analysis applied to single enzymes: can an isolated enzyme have a rate limiting step? Biochem. J. 294, 87–94.
Brown, G. C. and C. Cooper (1994). The analysis of rate limitation within enzymes: relations between flux control coefficients of rate constants and unidirectional rates, rate constants and thermodynamic parameters of single isolated enzymes. Biochem. J. 300, 159–164.
Burbaum, J. J., R. T. Raines, W. J. Albery and J. R. Knowles (1989). Evolutionary optimization of the catalytic effectiveness of an enzyme. Biochemistry 28, 9293–9305.
Chin, J. (1983). Perfect enzymes: is the equilibrium constant between the enzyme’s bound species unity. J. Am. Chem. Soc. 105, 6502–6503.
Demetrius, L. (1998). Role of enzyme-substrate flexibility in catalytic activity: an evolutionary perspective. J. Theor. Biol. 194, 175–194.
Heinrich, R. and E. Hoffmann (1991). Kinetic parameters of enzymatic reactions in states of maximal activity. An evolutionary approach. J. Theor. Biol. 151, 249–283.
Heinrich, R., E. Hoffmann and H.-G. Holzhütter (1990). Calculation of kinetic parameters of a reversible enzymatic reaction in states of maximal activity. Biomed. Biochim. Acta. 49, 891–902.
Heinrich, R. and T. A. Rappoport (1974). A linear steady state treatment of enzymatic chains. General properties. Control and effector strength. Eur. J. Biochem 42, 89–95.
Heinrich, R. and S. Shuster (1996). The Regulation of Cellular Systems, New York: Chapman and Hall.
Heinrich, R., S. Shuster and H.-G. Holzhütter (1991). Mathematical analysis of enzymatic reaction systems using optimization principles. Eur. J. Biochem. 201, 1–21.
Kacser, H. and J. A. Burns (1973). The control of flux. Symp. Soc. Exp. Biol. 27, 65–104.
Kacser, H. and R. Beeby (1984). Evolution of catalytic properties or the origin of enzyme species by means of natural selection. J. Mol. Evol. 20, 38–51.
Kholodenko, B. N. and H. V. Westerhoff (1994). Control theory of one enzyme. Biochim. Biophys. Acta. 1208, 294–305.
Klipp, E. and R. Heinrich (1999). Competition for enzymes in metabolic pathways: implications for optimal distributions of enzyme concentrations and for distribution of flux control. BioSystems 54, 1–14.
Knowles, J. R. and W. J. Albery (1977). Perfection in enzyme catalysis: the energetic of triosephophate isomerase. Acc. Chem. Res. 10, 105–111.
LoGrasso, P. V., C. Tu, D. A. Jewell, G. C. Wynns, P. J. Laipis and D. N. Silverman (1991). Catalytic enhancement of human carbonic anhydrase III by replacement of phenylalanine-198 with leucine. Biochemistry 30, 8463–8470.
Marin-Sanguino, A. and N. V. Torres (2000). Optimization of tryptophan production in bacteria. Design of a strategy for genetic manipulation of the tryptophan operon for tryptophan flux maximization. Biotechnol. Progress 16, 133–145.
Ni, T. C. and M. A. Savageau (1996). Model assessment and refinement using strategies from biochemical systems theory: application to metabolism in human red blood cells. J. Theor. Biol. 179, 329–368.
Orengo, C. A., D. T. Jones and J. M. Thornton (1994). Protein superfamilies and domain superfolds. Nature 372, 631–634.
Pettersson, G. (1992). Evolutionary optimization of the catalytic efficiency of enzymes. Eur. J. Biochem. 206, 289–295.
Regan, I., D. L. Bogle and P. Dunhill (1993). Simulation and optimization of metabolic pathways. Comput. Chem. Eng. 17, 627–637.
Savageau, M. A. (1976). Biochemical System Analysis: A Study of Function and Design in Molecular Biology, Reading, MA: Addison-Wesley.
Savageau, M. A. (1995). Michaelis-Menten mechanism reconsidered: implications of fractal kinetics. J. Theor. Biol. 176, 115–124.
Shiraishi, F. and M. A. Savageau (1992). The tricarboxylic acid cycle in dictiostelium discoideum. II. Evaluation of model consistency and robustness. J. Biol. Chem. 267, 22919–22925.
Sorribas, A. and M. A. Savageau (1989). Strategies for representing metabolic pathways within biochemical systems theory: reversible pathways. Math. Biosci. 94, 239–269.
Todd, A. E., C. A. Orengo and J. M. Thornton (1999). Evolution of protein function, from a structural perspective. Curr. Opin. Chem. Biol. 3, 548–556.
Torres, N. V., E. O. Voit and C. Gonzalez-Alcón (1996). Optimization of nonlinear biotechnological processes with linear programming: application to citric acid fermentation by Aspergillus niger. Biotechnol. Bioeng. 49, 247–258.
Torres, N. V., E. O. Voit, C. Gonzalez-Alcón and F. Rodriguez (1997). An integrated optimization method for biochemical systems. Description of method and application to ethanol, glycerol and carbohydrate production in Saccharomyces cerevisiae. Biotechnol. Bioeng. 55, 758–772.
Voit, E. O. (1992). Optimization of integrated biochemical systems. Biotechnol. Bioeng. 40, 572–582.
Voit, E. O. (2000). Computational Analysis of Biochemical Systems. A Practical Guide for Biochemists and Molecular Biologists, Cambridge: Cambridge University Press.
Wells, J. A. (1990). Additivity of mutational effects in proteins. Biochemistry 29, 8509–8517.
Wilhelm, T., E. Hoffman-Klipp and R. Heinrich (1994). An evolutionary approach to enzyme kinetics: optimization of ordered mechanisms. Bull. Math. Biol. 56, 65–106.
Zhou, G., M. T. Wong and G. Q. Zhou (1983). Diffusion controlled reactions of enzymes. Biophys. Chem. 18, 125–132.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marín-Sanguino, A., Torres, N.V. Modelling, steady state analysis and optimization of the catalytic efficiency of the triosephosphate isomerase. Bull. Math. Biol. 64, 301–326 (2002). https://doi.org/10.1006/bulm.2001.0276
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1006/bulm.2001.0276