Abstract
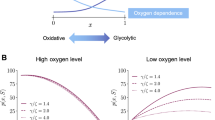
The tumour suppressor gene, p53, plays an important role in tumour development. Under low levels of oxygen (hypoxia), cells expressing wild-type p53 undergo programmed cell death (apoptosis), whereas cells expressing mutations in the p53 gene may survive and express angiogenic growth factors that stimulate tumour vascularization. Given that cells expressing mutations in the p53 gene have been observed in many forms of human tumour, it is important to understand how both wild-type and mutant cells react to hypoxic conditions.
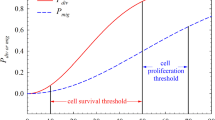
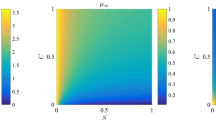
In this paper a mathematical model is presented to investigate the effects of alternating periods of hypoxia and normoxia (normal oxygen levels) on a population of wild-type and mutant p53 tumour cells. The model consists of three coupled ordinary differential equations that describe the densities of the two cell types and the oxygen concentration and, as such, may describe the growth of avascular tumours in vitro and/or in vivo. Numerical and analytical techniques are used to determine how changes in the system parameters influence the time at which mutant cells become dominant within the population. A feedback mechanism, which switches off the oxygen supply when the total cell density exceeds a threshold value, is introduced into the model to investigate the impact that vessel collapse (and the associated hypoxia) has on the time at which the mutant cells become dominant within vascular tumours growing in vivo.
Using the model we can predict the time it takes for a subpopulation of mutant p53 tumour cells to become the dominant population within either an avascular tumour or a localized region of a vascular tumour. Based on independent experimental results, our model suggests that the mutant population becomes dominant more quickly in vivo than in vitro (12 days vs 17 days).
Similar content being viewed by others
References
Adam, J. and J. C. Panetta (1995). A simple mathematical model and alternative paradigm for certain chemotherapeutic regimens. Math. Comput. Modelling 22, 49–60.
Anderson, A. R. A. and M. A. J. Chaplain (1998). Continuous and discrete mathematical models of tumour-induced angiogenesis. Bull. Math. Biol. 60, 857–900.
Baum, M., M. A. J. Chaplain, A. R. A. Anderson, M. Douek and J. S. Vaidya (1999). Does breast cancer exist in a state of chaos? Eur. J. Cancer 35, 886–891.
Byrne, H. M. and M. A. J. Chaplain (1996). Modelling the role of cell-cell adhesion in the growth and development of carcinomas. Math. Comput. Modelling 24, 1–17.
Carlsson, J. (1977). A proliferation gradient in three-dimensional colonies of cultured human glioma cells. Int. J. Cancer 20, 129–136.
Duchting, W., W. Ulmer and T. Ginsberg (1996). Cancer: A challenge for control theory and computer modelling. Eur. J. Cancer 32A, 1283–1292.
Ferreux, E., M. Wells and C. E. Lewis. Unpublished results.
Folkman, J. (1974). Tumour angiogenesis. Adv. Cancer Res. 19, 331–358.
Folkman, J. and M. Hochberg (1973). Self-regulation of growth in three dimensions. J. Exp. Med. 138, 745–753.
Freyer, J. P. (1988). Role of necrosis in regulating the growth saturation of multicellular spheroids. Cancer Res. 48, 2432–2439.
Gammack, D., H. M. Byrne and D. L. S. McElwain. A model of the effects of hypoxia and mutations in the p53 gene on tumour growth. In preparation.
Graeber, T. G., C. Ousmanian, T. Jacks, D. E. Housmann, C. J. Koch, S. W. Lowe and A. J. Giaccia (1996). Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature 379, 88–91.
Greenspan, H. P. (1974). On the growth and stability of cell cultures and solid tumours. J. Theor. Biol. 56, 229–242.
Gyllenberg, M. and G. F. Webb (1989). Quiescence as an explanation of Gompertzian tumour growth. Growth Dev. Aging 53, 25–33.
Hall, P. A. (1996). p53—integrating the complexity. J. Pathol. 180, 1–5.
Kinzler, K. W. and B. Vogelstein (1996). Life (and death) in a malignant tumour. Nature 379, 19–20.
Michelson, S. and J. T. Leith (1988). Unexpected equilibria resulting from differing growth rates of subpopulations within heterogeneous tumours. Math. Biosci. 91, 119–129.
Milner, J. (1996). The p53 tumour suppressor, in Signal Transduction, C.-H. Heldin and M. Purton (Eds), London: Chapman and Hall, pp. 335–346.
Muthakarrupan, V. R., L. Kubai and R. Auerbac (1982). Tumor-induced neovascularization in the mouse eye. J. Natl. Cancer Inst. 69, 699–705.
Panetta, J. C. (1997). A mathematical model of breast and ovarian cancer treated with paclitaxel. Math. Biosci. 146, 89–113.
Panetta, J. C. (1998). A mathematical model of drug resistance: heterogeneous tumours. Math. Biosci. 147, 41–61.
Royds, J. A., S. K. Dower, E. E. Qwarnstrom and C. E. Lewis (1998). Responses of tumour cells to hypoxia: role of p53 and NFkB. J. Clin. Pathol.: Mol. Pathol. 51, 55–61.
Sato, S., J. Kigawa, Y. Minagawa, M. Okada, M. Shimada, M. Takahashi, S. Kamazawa and N. Terakawa (1999). Chemosensitivity and p53-dependent apoptosis in epithelial ovarian carcinoma. Cancer 86, 1307–1313.
Sinik, Z., T. Alkibay, O. Ataoglu, H. Biri, S. Sözen, N. Deniz, Ü. Karaoglan and I. Bozkirli (1997). Nuclear p53 overexpression in bladder, prostate, and renal carcinomas. Int. J. Urol. 4, 546–551.
Sjögren, S., M. Inganös, T. Norberg, A. Lindgren, H. Norgren, L. Holmberg and J. Bergh (1996). The p53 gene in breast cancer: prognostic value of complementary DNA sequencing versus immunohistochemistry. J. Natl. Cancer Inst. 88, 173–181.
Thompson, K. E. and J. A. Royds (1999). Hypoxia and reoxygenation: a pressure for mutant p53 cell selection and tumour progression. Bull. Math. Biol. 61, 759–778.
Ward, J. P. and J. R. King (1999). Mathematical modelling of avascular-tumour growth II: modelling growth stauration. IMA J. Math. Appl. Med. Biol. 16, 171–211.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gammack, D., Byrne, H.M. & Lewis, C.E. Estimating the selective advantage of mutant p53 tumour cells to repeated rounds of hypoxia. Bull. Math. Biol. 63, 135–166 (2001). https://doi.org/10.1006/bulm.2000.0210
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1006/bulm.2000.0210