Abstract
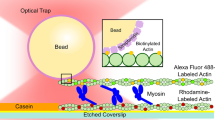
Optical trapping is one of the most evolving technologies that measures biophysical quantities and provides insights into some of the fundamental questions in the study of molecular motor proteins such as myosin. Several laboratories have successfully used this technique to observe and score nanometre-size displacements produced by myosin on interacting with actin. We have studied the distribution of attachment events for two myosin molecules with different orientations interacting with an actin filament within the framework of a Langevin-type bidirectional mathematical model. When myosin is detached from actin, our model predicts Brownian displacements centred at 0 ± 8 nm (mean ± SD, n = 251058). When attached, the time-averaged displacements of the actin filament system produced step sizes with peaks of 8 ± 6 nm (mean ± SD, n = 22174) (forward displacements) and −8 ± 6 nm (mean ± SD, n = 26769) (reverse displacements). We infer from our results that the population distribution of attachment events is strongly dependent on (i) the magnitude of the Brownian displacements, (ii) the location of the actin binding sites relative to the myosin molecules, (iii) the orientation of the myosin molcules, and (iv) the relative kinetics (rate constants) for the forward and reverse displacement events.
Similar content being viewed by others
References
Bentil, D. E., K. B. Acheampong, R. K. Wright and D. M. Warshaw (1997). Piconewton forces and nanometre steps: an assessment of experimental results for actomyosin interaction using Huxley kinetics. In Bio-Computing and Emergent Computation, Lundh, Olsson and Narayanan (Eds), London: World Scientific Press, pp. 228–237.
Bentil, D. E. (1998a). Modeling and simulation of motility in actomyosin systems. J. Comp. Biol. 5, 73–86.
Bentil, D. E. (1998b). How Brownian displacement goes down during actin-myosin interaction in the laser trap. Math. Comp. Modelling. (in press).
Berg, H. C. (1983). Random Walks in Biology, New Jersey: Princeton University Press.
Brokaw, C. J. (1976). Computer simulation of movement-generating cross-bridges. Biophys. J. 16, 1013–1027
Chandrasekhar, S. (1943). Stochastic problems in physics and astronomy. Rev. Mod Phys. 15, 1–89.
Córdova, N. J., B. Ermentrout and G. F. Oster (1992). Dynamics of single-motor molecules: the thermal ratchet model. Proc. Natl. Acad. Sci. USA 89, 339–343.
Dupuis, D. E., W. H. Guilford, J. Wu and D. M. Warshaw (1997). Actin filament mechanics in the laser trap. J. Musl. Res. Cell Mot. 18, 17–30.
Finer, J., R. M. Simmons and J. A. Spudich (1994). Single myosin molecule mechanics: piconewton forces and nanometre steps. Nature 368, 113–118.
Finer, J. T., A. D. Mehta and J. A. Spudich (1995). Characterization of single actin-myosin interactions. Biophys. J. 68, 291s–297s.
Guilford, W. H., D. E. Dupuis, G. Kennedy, J. Wu, J. B. Patlak and D. M. Warshaw (1997). Smooth-muscle and skeletal-muscle myosins produce similar unitary forces and displacements in laser trap. Biophys. J. 72, 1006–1021.
Hill, T. L. (1974). Theoretical formalism for the sliding filament model of contraction of striated muscle. Prog. Biophys. Mol. Biol. 28, 267–340.
Huxley, A. F. (1957). Muscle Structure and theories of contraction. Prog. Biophys. 7, 255–318.
Ishijima, A., T. Doi, K. Sakurada and T. Yanagida (1991). Sub-piconewton force fluctuations of actomyosin in vitro. Nature 352, 301–306.
Ishijima, A., Y. Harada, H. Kojima, T. Funatsu, H. Higuchi and T. Yanagida (1994). Single-molecule analysis of the actomyosin motor using nano-manipulation. Biochem. Biophys. Res. Comm. 199, 1057–1063.
Miyata, H., H. Hakozaki, H. Yoshikawa, N. Suzuki, K. Kinosita Jr., T. Nishizaka and S. Ishiwata (1994). Stepwise motion of an actin filament over a small number of heavy meromyosin molecules is revealed in an in vitro motility assay. J. Biochem. 155, 644–647.
Molloy, J. E., J. E. Burns, J. Kendrick-Jones, R. T. Tregear and D. C. White (1995a). Movement and force produced by a single myosin head. Nature 378, 209–212.
Molloy, J. E., J. E. Burns, J. C. Sparrow, R. T. Tregear, J. Kendrick-Jones and C. S. White (1995b). Single-molecule mechanics of heavy meromyosin and s1 interacting with rabbit or drosophila actin using optical tweezers. Biophys. J. 68, 298s–350s.
Molloy, J. E. and C. S. White (1997). Smooth and skeletal muscle single-molecule mechanical experiments. Biophys. J. 72, 984–986.
Purcell, E. M. (1977). Life at low Reynolds number. Am. J. Phys. 45, 3–11.
Reedy, M. K., K. C. Holmes and R. T. Tregear (1965). Induced changes in orientation of the crossbridges of glycerinated insect flight muscle. Nature 207, 1276–1280.
Reedy, M. K. (1967). Crossbridges and periods in insect flight muscle. Am. Zool. 7, 465–481.
Riskin, H. (1989). The Fokker-Plank Equation, New York: Springer.
Schneider, T. and E. Stoll (1978). Molecular-dynamics study of a three-dimensional one-component model for distortive phase transitions. Phys. Rev. B 17, 1302–1322.
Sellers, J. R. and B. Kachar (1990). Polarity and velocity of sliding filaments: control of direction by actin and of speed by myosin. Science 249, 406–408.
Sheetz, M. P. and J. A. Spudich (1983). Movement of myosini-coated fluorescent beads on actin cables in vitro. Nature 303, 31–35.
Svoboda, K. and S. M. Block (1994). Biological applications of optical forces. Ann. Rev. Biophys. Biomol. Struct. 23, 247–285.
Svoboda, K., C. F. Schmidt, B. J. Schnapp and S. M. Block (1993). Direct observation of kinesin stepping by optical trapping interferometry. Nature 365, 721–727.
Toyoshima, Y. Y., C. Toyoshima and J. A. Spudich (1989). Bidirectional movement of actin filaments along tracks of myosin heads. Nature 341, 154–156.
VanBuren, P., S. S. Work and D. M. Warshaw (1994). Enhanced force generation by smooth muscle myosin in vitro. Proc. Natl. Acad. Sci. USA 91, 202–205.
Yamada, A., N. Ishii and K. Takahashi (1990). Direction and speed of actin filaments moving along thick filaments isolated from molluscan smooth muscle. J. Biochem. 108, 341–343.
Yamada, A. and K. Takahashi (1992). Sudden increase in speed of an actin filament moving on myosin crossbridges of “mismatched” polarity observed when its leading end begins to interact with crossbridges of matched polarity. J. Biochem. 111, 676–680.
Yamada, A. and T. Wakabayashi (1993). Movement of actin away from the center of reconstituted rabbit myosin filament is slower than in the opposite direction. Biophys. J. 64, 565–569.
Yanagida, T., M. Nakase, K. Nishiyama and F. Oosawa (1984). Direct observation of single F-actin filaments in the presence of myosin. Nature 307, 58–60.
Warshaw, D. M. (1996). The in vitro motility assay: a window into the myosin molecular motor. News Physiol. Sc. 11, 1–7.
West, J. M., H. Higuchi, A. Ishijima and T. Yanagida (1996). Modification of the bi-directional sliding movement of actin filaments along native thick filaments isolated from a clam. J. Musl. Res. Cell Mot. 17, 637–646.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bentil, D.E. Distribution of attachment events relative to actin binding sites as evidenced in a bidirectional actomyosin interaction model. Bull. Math. Biol. 60, 973–995 (1998). https://doi.org/10.1006/bulm.1998.0055
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1006/bulm.1998.0055