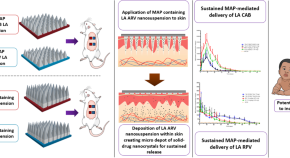
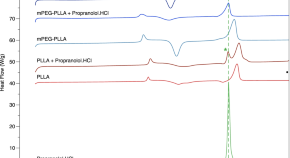
Current State and Opportunities with Long-acting Injectables: Industry Perspectives from the Innovation and Quality Consortium “Long-Acting Injectables” Working Group
Authors (first, second and last of 21)

Collection
Dr. Barrett is a Senior Principal Scientist at Merck & Co., Inc., Rahway, NJ and is one of Merck’s leading experts in polymer and bioconjugate chemistry for enabled delivery of small molecule and macromolecular drugs, having made influential contributions to the discovery of novel polymer conjugates for siRNA therapeutics, polymer excipients for oral dosage forms, and polymer-based long-acting injectables and implantables. She is currently leading the team responsible for the design of Merck’s next generation of long-acting implant and injectable products—with potential applications in Neuroscience, HIV and other infectious diseases.
Filippos Kesisoglou, PhD, is a Distinguished Scientist at Merck & Co., Inc., Rahway, NJ and had been leading the translational biopharmaceutics efforts across the small and large molecule portfolio in the Pharmaceutical Sciences department. He has more than 15 years of experience in the fields of biopharmaceutics and formulation development, PBPK and IVIVC modeling as related to clinical, drug product development and CMC regulatory applications. He has contributed to numerous new drug applications along therapeutic areas.