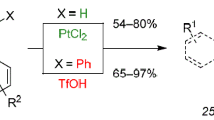
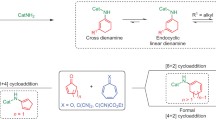
Abstract
The synthetic potential of the intramolecular ketene cycloaddition is presented. It proves to be a versatile method for the synthesis of a variety of bi- and tricyclic cyclobutanones. The major side reaction, namely the dimerization of the ketene is prevented by the introduction of conformational restrictions. This has been achieved by an additional double bond or by an annulated ring system. Following this strategy C-analogues of a penem and a carbapenem as well as linear and angular annulated triquinanes are obtained in high yield.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
REFERENCES
H. Staudinger, Chem. Ber. 44 (1911) 521.
H.K. Hal1, D.E. Plorde, US-Pat. 3646150 (1968), Du Pont.
W.T. Brady, O.H. Waters, J. Org. Chem. 32 (1967) 3703.
J.B. Sieja, J. Am. Chem. Soc. 93 (1971) 130.
R.R. Sauers, K.W. Kelly, J. Org. Chem. 35 (1970) 3286.
J.J. Beereboom, J. Am. Chem. Soc. 85 (1963) 3525; J. Org. Chem. 30 (1963) 4230
W.F. Erman, J. Am. Chem. Soc. 91 (1969) 779
S.W. Baldwin, E.H. Page, J. Chem. Soc. Chem. Commun. 1972, 1337
F. Leyendecker, R. Bloch, J.M. Conia, Tetrahedron Lett. 1972, 3703
R.H. Bisceglia, C.J. Cheer, J. Chem. Soc. Chem. Commun. 1973, 165
S. Moon, T.F. Kolesar, J. Org. Chem. 39 (1974) 995
L. Libit, US-Pat. 4005109 (1974)
A. Smit, J.G.J. Kok, H.W. Geluk, J. Chem. Soc. Chem. Commun. 1975, 513
F. Leyendecker, Tetrahedron 32 (1976) 349
T. Sasaki, S. Eguchi, Y. Hirako, J. Org. Chem. 42 (1977) 2981
A. Maujean, G. Marcy, J. Cuche, J. Chem. Soc. Chem. Commun. 1980, 92
M. Kuzuya, F. Miyake, T. Okuda, Tetrahedron Lett. 1980, 1043 and 2185
A. Alder, D. Bellus, J. Am. Chem. Soc. 105 (1983) 6712
H. Dhimane, J.C. Pommelet, J. Cuche, Tetrahedron Lett. 26 (1985) 833
F. Arya, J. Bouquant, J. Cuche, Tetrahedron Lett. 27 (1986) 1913.
W.F. Erman, J. Am. Chem. Soc. 89 (1967) 3828
O.L. Chapman, J.D. Lassila, J. Am. Chem. Soc. 90, (1968) 2449
O.L. Chapman, M. Kane, J.D. Lassila, R.L. Loeschen, H.E. Wright, J. Am. Chem. Soc. 91 (1969) 6856
A.S. Kende, Z. Goldschmit, P.T. Izzo, J. Am. Chem. Soc. 91 (1969) 6858
H. Hart, G.M. Lowe, J. Am. Chem. Soc. 93 (1971) 6266
D. Becker, M. Nagler, D. Birnbaum, J. Am. Chem. Soc. 94 (1972) 4771
Z. Goldschmit, U. Gutman, Y. Bakal, A. Worchel, Tetrahedron Lett. 1973, 3759
D. Becker Z. Harel, D. Birnbaum, J. Chem. Soc. Chem. Commun. 1975, 377
S. Ayral-Kaloustian, S. Wolff, W.C. Agosta, J. Org. Chem. 43 (1978) 3314
D. Becker, D. Birnbaum, J. Org. Chem. 45 (1980) 570
R.E. Ireland, J.D. Godfrey, S. Thaisrivongs, J. Am. Chem Soc. 103 (1981) 2446
E. Lee-Ruff, A.C. Hopkinson, H. Kazarians-Moghaddam, Tetrahedron Lett. 24 (1983) 2067
A.G. Schultz, J.P. Dittami, K.K. Eng, Tetrahedron Lett. 25 (1984) 255.
L. Ghosez, M.J. O’Donnell in Pericyclic Reactions, A.P. Marchand R.E. Lehr, Eds., Academic Press, New York, 1977, Vol. 2, p. 79–140.
M. Kuzuya, F. Miyake, T. Okuda, J. Chem. Soc. Perkin Trans. II, 1984, 1471
E. Sonveaux, J.M. Andre, J. Delhalle, J.G. Fripiat, Bull. Soc. Chim. Belg. 94, (1985) 831.
G.B. Payne, J. Org. Chem. 31 (1966) 718.
I. Markó, B. Ronsmans, A.-M. Hesbain-Frisque, S. Dumas, L. Ghosez, B. Ernst, H. Greuter, J. Am. Chem. Soc. 107 (1985) 2192.
I. Fleming in Frontier Orbitals and Organic Chemical Reactions, J. Wiley & Sons, 1976, 143 ff.
Y.S. Kulkarni, B.B. Snider, J. Org. Chem. 50 (1985) 2809
Y.S. Kulkarni, B.W. Burbaum, B.B. Snider, Tetrahedron Lett. 26 (1985) 5619
B.B. Snider, E. Ron, B.W. Burbaum, J. Org. Chem. 52 (1987) 5413.
E.J. Corey, M.C. Desai, Tetrahedron Lett. 26 (1985) 3535.
M.I. Page, Acc. Chem. Res. 17 (1984) 144.
E.M. Gordon, J. Pluščec, M.A. Ondetti, Tetrahedron Lett. 22 (1981) 1871
G.A. Boswell, Jr., A.J. Cocuzza, US-Pat. 4 505 905 (1982), Du Pont
O. Meth-Cohn, A.J. Reason, S.M. Roberts, J. Chem. Soc. Chem. Commun. 1982, 90
G. Lowe, S. Swain, ibid. 1983, 1279
A.J. Cocuzza, G.A. Boswell, Tetrahedron Lett. 26 (1985) 5363
G. Lowe, S. Swain, J. Chem. Soc., Perkin Trans, I 1985, 391
G. Lange, M.E. Savard, T. Viswanatha, G.I. Dmitrienko, Tetrahedron Lett. 26 (1985) 1791
D. Agathocleous, G. Cox, M.I. Page, Tetrahedron Lett. 27 (1986) 1631.
H. Muruyama, T. Hiraoka, J. Org. Chem. 51 (1986) 399 and references cited herein.
Prepared by Dr. P. Schneider, Pharmaceuticals Division, CIBA-GEIGY Ltd., CH-4002 Basel.
A. Wissner, C.V. Grudzinskas, J. Org. Chem. 43 (1978) 3972.
The analogous reaction with vinyl lithium was described: C.L. Semmelhack, I.-C. Chiu, K.G. Grohman, J. Am. Chem. Soc. 98 (1976) 2005.
B. Haveaux, A. Dekoker, M. Rens, A.R. Sidani, J. Toye, L. Ghosez, Org. Synth. 59, 26.
L. Lombardo, Tetrahedron Lett. 26 (1985) 381.
J.E. Baldwin, M.C. McDaniel, J. Am. Chem. Soc. 89 (1967) 1537
J.E. Baldwin, M.C. McDaniel, J. Am. Chem. Soc. 90 (1968) 6118.
H. Mayr, R. Huisgen, Ang. Chem. 87 (1975) 491.
C. Exon, P. Magnus, J. Am. Chem. Soc. 105 (1983) 2477 and references cited therein.
R.L. Funk, G.L. Bolton, J. Org. Chem. 49 (1984) 5021 and references cited therein.
L.A. Paquette, Y.-K. Han, J. Am. Chem. Soc. 103 (1981) 1835
P.A. Wender, G.B. Dreyer, Tetrahedron 37 (1981) 4445
M.C. Pirrung, J. Am. Chem. Soc. 103 (1981) 82 and references cited therein.
G.D. Annis, L.A. Paquette, J. Am. Chem. Soc. 104 (1982) 4504.
H. Schostarez, L.A. Paquette, J. Am. Chem. Soc. 103 (1981) 722.
A. De Mesmaeker, S.J. Veenstra, B. Ernst, Tetrahedron Lett. 29 (1988) 459.
The allylcyclopentanone 77 was prepared in three steps from cyclopentanone via allylation of the corresponding dimethylhydrazone (overall yield 72 %) in analogy with E.J. Corey, D. Enders, Tetrahedron Lett. 1976, 3.
For deconjugation of α,b-unsaturated esters during Horner- Emmons and Peterson olefination see
S.L. Hartzell, D.F. Sullivan, M.W. Rathke, Tetrahedron Lett. 1974, 1403
H. Taguchi, Soc. Jpn. 47 (1974) 2529.
J.R. Stille, R.H. Grubbs, J. Am. Chem. Soc. 108 (1986) 855.
S.J. Veenstro, A. De Mesmaeker, B. Ernst, Tetrahedron Lett. 29 (1988) 2303.
P. Beak, D.J. Kempf, K.D. Wilson, J. Am. Chem. Soc. 107 (1985) 4745.
J.S. Yadav, B.V. Joshi, V.R. Gadgil, Indian J. Chem., Sect. B 26 (1987) 399.
The autors thank Dr. D. Poppinger, Zentrale Funktion Forschung, Computer Chemistry, Ciba-Geigy Ltd., CH-4002 Basel for the calculations according to: M.J.S. Dewar, E.G. Zoebisch, E.F. Healy, J.J.P. Stewart, J. Am. Chem. Soc. 107 (1985) 3902; Quantum Chemistry Program Exchange Nr. 506.
H. Marschal1, F. Vogel, Chem. Ber. 107 (1974) 2176.
Y. Fukuda, T. Negoro, Y. Tobe, K. Kimura, Y. Odaira, J. Org. Chem. 44 (1979) 4557.
L. Ghosez, I. Markó, A.-M. Hesbain-Frisque, Tetrahedron Lett. 27 (1986) 5211.
B.B. Snider, R.A.H.F. Hui, Y.S. Kulkarni, J. Am. Chem. Soc. 107 (1985) 2194
B.B. Snider, R.A.H.F. Hui, J. Org. Chem. 50 (1985) 5167.
W.T. Brady, Y.F. Giang, J. Org. Chem. 50 (1985) 5177
W.T. Brady, Y.F. Giang, A.P. Marchand, A.H. Wu, J. Org. Chem. 52 (1987) 3457
W.T. Brady, A.P. Marchand, Y.F. Giang, A.H. Wu, Synthesis 1987 395.
E.J. Corey, M.C. Desai, T.A. Engler, J. Am. Chem. Soc. 107 (1985) 4339.
W. Oppolzer, A. Nakao, Tetrahedron Lett. 27 (1986) 5471.
H.-U. Reisig, Nachr. Chem. Tech. Lab. 34 (1986) 880.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1989 Kluwer Academic Publishers
About this chapter
Cite this chapter
Ernst, B., de Mesmaeker, A., Greuter, H., Veenstra, S.J. (1989). Intramolecular [2+2] Cycloadditons of Ketenes and Olefins. In: de Meijere, A., Blechert, S. (eds) Strain and Its Implications in Organic Chemistry. NATO ASI Series, vol 273. Springer, Dordrecht. https://doi.org/10.1007/978-94-009-0929-8_14
Download citation
DOI: https://doi.org/10.1007/978-94-009-0929-8_14
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-010-6907-6
Online ISBN: 978-94-009-0929-8
eBook Packages: Springer Book Archive