Summary
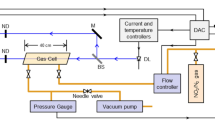
A Tunable Diode Laser Spectrometer (TDLS) has been developed and used to measure hydroperoxy compounds in atmospheric mixing ratios and to study their chemical behaviour under atmospheric conditions. The TDLS was coupled to a White cell which allows in combination with a 2f technique, the measurement of H202 mixing ratios below 0.2 ppb. The 2f signal was calibrated against H2O2 concentrations via UV absorption and checked by comparison with a peroxyoxalate chemiluminescence method. The reactions of some biogenic alkenes with ozone were studied under atmospheric conditions to determine the yield of H2O2 formed in these systems.
The absorption cross-sections of methylhydroperoxide near 1320 cm-1 were also measured in order to assess the possibility of its determination in the atmosphere.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Lind, I.A., Lazrus, A.L. and Kok, G.L. (1987); Aqueous phase oxidation of S (IV) by hydrogen peroxide, methylhydroperoxide and peroxyacetic acid; J. Geophys. Res. Vol. 92, 4171 – 4177
Calvert, I.G. et al., (1985), Chemical mechanisms of acid generation in the troposphere; Nature Vol. 317, 27–35
Lind, J.A. and Kok, G.L., (1986); Henry’s law constants of hydrogen peroxide, methylhydroperoxide and peroxyacetic acid; J. Geophys Res. Vol. 91, 7889–7895
Jakob, P. and Klockow, D.; privat communication
Molina, L.T., Schinke, D., Molina, M.J., (1977); UV absorption spectra of hydrogen peroxide vapor; Geophys. Res. Lett. Vol. 4, 580 – 582
Jakob, P., Tavares, T.T. and Klockow, D.,(1986); Metodology for the determination of gaseous hydrogen peroxide in ambient air; Z. Anal. Chemie Vol. 325, 359–365
Rieche, A. and Hitze, F. (1929), Über Methylhydroperoxid; Ber. Dtsch. Chem. Ges. B. Vol. 62, 2458 – 2474
Niki, H., Maker, P.D.and Savage, C.M. (1983), FTIR study of the kinetics and mechanism for the reaction HO + CH3OOH; J. Phys. Chem. Vol. 87, 2190 – 2193
Molina, M.J. and Arguello, G. (1979); UV absorption spectra of methylhydroperoxide vapor; Geophys. Res. Lett. Vol 6, 953 – 955
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1990 ECSC, EEC, EAEC, Brussels and Luxembourg
About this chapter
Cite this chapter
Bechara, J., Becker, K.H., Brockmann, K.J. (1990). Studies on the spectroscopy and chemistry of some atmospheric hydroperoxides with a tunable diode laser. In: Restelli, G., Angeletti, G. (eds) Physico-Chemical Behaviour of Atmospheric Pollutants. Springer, Dordrecht. https://doi.org/10.1007/978-94-009-0567-2_4
Download citation
DOI: https://doi.org/10.1007/978-94-009-0567-2_4
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-010-6743-0
Online ISBN: 978-94-009-0567-2
eBook Packages: Springer Book Archive