Abstract
Sperm chemotaxis is widely seen both in animals and plants and is considered to be necessary for efficient success of fertilization. Although intracellular Ca2+ is known to play important roles in sperm chemotaxis, the molecular mechanism causing the change in flagellar waveform that drives sperm directed toward the egg is still unclear. Several Ca2+-binding proteins, especially calmodulin, have been discussed as an important regulator of the molecular motor dynein in flagellar motility during chemotactic movement of sperm. However, there has been no experimental evidence to show the binding of calmodulin to dyneins. Recently, we found a novel Ca2+-binding protein, termed calaxin, in the axonemes of sperm flagella in the ascidian Ciona intestinalis. Calaxin binds to the outer arm dynein in a Ca2+-dependent manner and suppresses its activity to slide microtubules at high Ca2+ concentration. Inhibition of calaxin results in significant loss of chemotactic behavior of sperm, indicating that calaxin is essential for sperm chemotaxis. In this chapter, we describe the finding history, molecular nature, and the roles in sperm chemotaxis of calaxin, as well as its phylogenetic consideration.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Ca2+ and Flagellar Motility
The flagellar wave is composed of a bend with larger angle called the principal bend (P-bend) and a bend with a smaller angle called the reverse bend (R-bend). Sperm showing the same extent of both bends show symmetrical waveform and swim straight, whereas a decrease in R-bend results in asymmetry of waveform and the circular swimming of sperm (Fig. 5.1). This waveform conversion is conducted by the regulation of dynein-driven axonemal motility. It has been well known that Ca2+ plays an important role in the regulation of the axonemal motility in eukaryotic flagella and cilia (Kamiya and Witman 1984; Gibbons and Gibbons 1980; Sale 1986). In fact, increasing the concentration of Ca2+ in Triton-demembranated sperm induces conversion of the flagellar waveform from symmetry to asymmetry (Brokaw 1979). An extremely asymmetrical waveform is induced at very high calcium concentrations (Gibbons and Gibbons 1980; Sale 1986). Sperm with this waveform show cane-shaped “quiescence,” which is observed by electric or mechanical stimulation of sperm flagella in the sea urchin (Shingyoji and Takahashi 1995; Kambara et al. 2011). On the self-nonself recognition of sperm and egg in Ciona, sperm show quiescence with a straight flagellum in response to the increase in intracellular Ca2+ (Saito et al. 2012). The basic regulation of flagellar waveform by Ca2+ is thought to be performed by the specific activation of dynein arms, which results in the changes in flagellar waveforms (Brokaw 1979; Lindemann and Goltz 1988). Accumulating evidence indicates that the activity of inner arm dyneins is regulated by signals from the radial spoke/central pair in a Ca2+-dependent manner (Smith 2002; Nakano et al. 2003). On the other hand, independent regulation of the outer arm dynein by Ca2+ is also pointed out (Mitchell and Rosenbaum 1985; Wakabayashi et al. 1997; Sakato and King 2003).
Flagellar waveform and the direction of sperm movement. The flagellar wave is composed of a large principal bend (P-bend) and a smaller reverse bend (R-bend). Sperm with almost the same extent of P-bend and R-bend move straight, whereas those with larger R-bend move in circular fashion. Inverse of the radius of inscribed circle represents curvature, which is a parameter to express the extent of flagellar bending
Calmodulin has been a strong candidate to regulate the conversion of flagellar and ciliary waveform. In fact, several studies have discussed the presence and potential roles of calmodulin in Tetrahymena cilia (Jamieson et al. 1979; Blum et al. 1980), Chlamydomonas flagella (Gitelman and Witman 1980), and sperm flagella (Tash and Means 1983; Brokaw and Nagayama 1985; Lindemann et al. 1991). It is well demonstrated that calmodulin is present in radial spokes and central pair and regulates the function of these structures in the modulation of axonemal dyneins in Chlamydomonas (Smith and Yang 2004). Another Ca2+-binding protein, centrin, is known to be a component of inner arm dynein (Piperno et al. 1992).
In contrast, analysis of Chlamydomonas mutants indicates that outer arm dyneins are essential for conversion of waveform asymmetry in response to changes in Ca2+ concentration (Kamiya and Okamoto 1985; Wakabayashi et al. 1997; Sakato and King 2003). In fact, a Ca2+-binding protein is contained in the outer arm dynein as a light chain (LC4) (King and Patel-King 1995). Another Ca2+-binding protein associated with the outer arm dynein in Chlamydomonas flagella is DC3, a component of outer arm dynein docking complex (ODA-DC) (Casey et al. 2003). DC3 protein is structurally distinct from other Ca2+-binding proteins in Chlamydomonas flagella, such as calmodulin, centrin, and LC4. Intriguingly this protein shows sequence similarity to a protein predicted in the Apicomplexa Plasmodium yoelii and Plasmodium falciparum (Casey et al. 2003), but its orthologue has not been found in the genome of the ascidian Ciona intestinalis (Hozumi et al. 2006). Thus, the Ca2+-binding protein that regulates axonemal dyneins had not been fully characterized in sperm flagella. Calmodulin was reported to regulate flagellar motility and could be extracted from axonemes with outer arm dynein, but it has not been clarified whether calmodulin can bind directly to outer arm dynein (Tash et al. 1988). In fact, isolated outer arm dynein does not contain calmodulin as a subunit in Ciona and sea urchin (Inaba 2007).
2 Finding Calaxin
During the course of immunoscreening-based cDNA screening for axonemal proteins in C. intestinalis, we isolated multiple clones from testis cDNA library encoding a protein with sequence similarity to calcineurin B (Padma et al. 2003). Phylogenetic analysis revealed that this protein is grouped not into calcineurin B but into a family of neuronal calcium sensor (NCS). We named this novel Ca2+-binding NCS family protein in Ciona as “calaxin,” for calcium-binding axonemal protein (Mizuno et al. 2009).
NCS proteins have been identified in many organisms ranging from yeast to human (Burgoyne and Weiss 2001). Major five classes of NCS have been well studied in human: NCS-1 (frequenin), neurocalcin and its related proteins (visinin-like protein VILIP and hippocalcin), recoverin, GCAP (guanylyl cyclase-activating protein), and KChIP (Kv channel-interacting protein) (Burgoyne 2004). In mammals, recoverin and GCAPs are expressed only in the retina and regulate phototransduction and others are expressed in neuronal tissues. NCS-1 is also expressed in many nonneuronal cell types, and its orthologue is present in yeast. The NCS proteins contain four EF hand motifs but only three (or two in the case of recoverin and KChIP1) are able to bind Ca2+. Eleven of 15 mammalian NCS proteins are N-terminally myristoylated, which are important in Ca2+-dependent interaction with the plasma membrane. In contrast to these NCS proteins, calaxin does not possess the N-terminal consensus motif for myristoylaion and belongs to a class distinct from these five NCS classes (Mizuno et al. 2009).
Immunolocalization reveals that calaxin is localized at the vicinity of the outer arm dyneins (Mizuno et al. 2009). Sucrose density gradient centrifugation clearly indicates that calaxin directly interacts with the outer arm dynein in a Ca2+-dependent manner. Far Western blotting and a cross-linking experiment show that calaxin binds to the β-heavy chain in the presence of Ca2+, whereas it binds to β-tubulin in both the presence and absence of Ca2+. Preliminary experiments showed that calaxin binds to the N-terminal stem region of β-heavy chain (Mizuno, unpublished observation).
Although a phylogenetic analysis shows that Chlamydomonas LC4 and Ciona calaxin are grouped into different classes of Ca2+-binding protein, there are many similarities between them (Sakato and King 2003; Sakato et al. 2007; Mizuno et al. 2009; 2012). First, they appear to undergo dynamic conformational change in response to Ca2+ binding. Second, their binding sites are the stems of specific dynein heavy chains: γ-heavy chain of Chlamydomonas outer arm dynein for LC4 and its orthologue in Ciona, β-heavy chain for calaxin. Third, they mediate binding between dynein and microtubules. Regardless of these common properties, calaxin exhibits characteristic features: Ca2+-dependent binding to dynein heavy chain and Ca2+-independent binding to β-tubulin (and possibly to intermediate chain 2 [IC2], orthologue of Chlamydomonas IC1). Because Ciona lacks both LC4 and DC3, calaxin might be evolved to play double roles of LC4/DC3 in Ca2+-dependent regulation of outer arm dynein (also see next section).
3 Mechanism of Calaxin-Mediated Modulation of Flagellar Movements During Sperm Chemotaxis
During chemotaxis in Ciona, sperm repeat straight and turn movements to come toward the egg (Fig. 5.2). The turn movement accompanies transient increase in intracellular Ca2+ concentration and asymmetry of flagellar waveform (Shiba et al. 2008). It was not been elucidated how the increase in intracellular Ca2+ concentration induced the modulation of flagellar bending during sperm chemotaxis. As we previously showed that calaxin was directly bound to the outer arm dynein in a Ca2+-dependent manner, it was a strong candidate for direct Ca2+-dependent modulator for flagellar waveform of sperm (Mizuno et al. 2009). Localization of calaxin to epithelial cilia also suggests a possibility that calaxin is a general Ca2+ sensor to modulate ciliary and flagellar motility (Mizuno et al. 2009).
An antidiabetic compound, repaglinide, is a specific inhibitor for NCS and is also effective on calaxin (Okada et al. 2003; Mizuno et al. 2012). In the presence of repaglinide, sperm do not show the unique turn movement, resulting in less effective chemotaxis. Flagellar bending with strong asymmetry continues for ~0.1 ms during one turn in normal sperm. Repaglinide-treated sperm exhibit transient flagellar asymmetry, but the asymmetry is not sustained for long, and sperm exhibit only incomplete turning (Mizuno et al. 2012). Thus, it is suggested that calaxin plays a key role in sustaining the asymmetrical waveform, not in its formation (Mizuno et al. 2012).
In a demembranated sperm model treated with repaglinide, flagellar bending becomes attenuated at a high concentration of Ca2+. This attenuation is not observed at low concentration of Ca2+, suggesting that calaxin regulates dynein-driven microtubule sliding for asymmetrical bending at higher Ca2+ concentration (<10−6 M). By using an in vitro assay system with purified dynein, microtubules, and calaxin, the roles of calaxin in the regulation of dynein-driven microtubule sliding can be directly examined (Mizuno et al. 2012). Increasing the concentration of Ca2+ has a small effect on the velocity of microtubule sliding by Ciona outer arm dynein. Addition of calaxin gives no significant change in the sliding. On the other hand, at higher Ca2+ concentrations (<10−6 M), addition of calaxin significantly reduced the velocity of microtubule translocation. Thus, calaxin is thought to bind and suppress outer arm dynein at high concentrations of Ca2+. This suppression is thought necessary for the propagation of asymmetrical bending.
The mechanism of calaxin-mediated chemotactic turn is summarized in Fig. 5.3. Before the chemotactic turn, Ca2+ concentration in sperm is low and the calaxin is dissociated from dynein. Symmetric P- and R-bends are properly propagated, resulting in straight swimming of sperm. When intracellular Ca2+ concentration is raised by Ca2+ influx, calaxin suppresses dynein-driven microtubule sliding, resulting in propagation of asymmetrical bending and turn movement of sperm. After the chemotactic turn, sperm show straight movement. Therefore, calaxin is thought to be again dissociated from dynein and sperm swim straight with a symmetrical flagellar waveform. Ca2+ imaging of live Ciona sperm, however, demonstrates that intracellular Ca2+ concentration is still high just after the chemotactic turn (Shiba et al. 2008). It is possible that binding of calaxin to dynein is controlled not by absolute Ca2+ concentration but by the difference in Ca2+ concentration. Alternatively, calaxin may be downregulated by some factors after the chemotactic turn. The mechanism of calaxin after the chemotactic turn is still to be elucidated.
Molecular events during chemotactic turn movement of Ciona sperm shown by 16 sequential images of sperm waveform during chemotactic turn. The 8 images highlighted in the center represent turn with asymmetrical waveform. The molecular events during this process are driven by the changes of intracellular Ca2+ and interaction between calaxin and dynein (see text for more detail)
4 A Phylogenetic Consideration of Calaxin
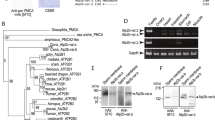
Homology search against databases of other organisms demonstrates that calaxin orthologues are present in vertebrates, such as human, mouse and Xenopus. Calaxin is also found in invertebrates, both deuterostome (Ciona, lancet, and sea urchin) and protostome (Drosophila). Search against genome databases of the sea anemone Nematostella vectensis and the choanoflagellate Monosiga brevicollis also identifies calaxin orthologues in these organisms (Mizuno et al. 2009). However, calaxin has not been found in yeast, Volvox, Trypanosoma, or Arabidopsis, implying that calaxin is metazoan specific (Mizuno et al. 2009). Recently, high-throughput next-generation sequencing enables us to determine draft sequences from a number of other organisms. We have recently found a calaxin orthologue in the chytrid fungus Batrachochytrium dendrobatidis (Fig. 5.4). Further search against genome databases of other organisms supports the idea that calaxin is an opisthokont-specific protein to regulate axonemal dyneins (Inaba et al., manuscript in preparation).
Multiple alignment of calaxin. Sequences of calaxin from Homo sapiens (Hs), Ciona intestinalis (Ci), and Batrachochytrium dendrobatidis (Bd) are aligned by ClustalW. Asterisks, colons, or dots indicate identical residues in all sequences in the alignment, conserved substitutions, or semi-conserved substitutions, respectively
Ca2+-dependent regulation of flagellar waveform is important for responses of organisms to several stimuli. For example, Chlamydomonas exhibits several light-induced behavioral responses, including phototaxis, photophobic response, and photokinesis (Witman 1993; Wakabayashi and King 2006). Paramecium swims both backward and forward according to the changes in intracellular Ca2+ (Naitoh and Kaneko 1972). The outer arm dynein of Paramecium cilia has not been well characterized, but a gene for the orthologue of Chlamydomonas LC4 or DC3 is found in the Paramecium genome. On the other hand, neither LC4 nor DC3 is found in Ciona, as already described. Considering the regulation of the outer arm dynein by Ca2+ commonly seen in Chlamydomonas, Paramecium, and Ciona, it is possible to consider that calaxin is an opisthokont-specific innovation for Ca2+-dependent regulation of axonemal dyneins.
5 Perspectives
Calaxin was first identified in Ciona sperm but was found to be distributed all through opisthokonts. Considering the presence of Ca2+-dependent regulator for dynein, LC4, in Chlamydomonas and other bikont species, what does this “innovation of calaxin” in opisthokonts mean? It is possible that an unknown mechanism for motility regulation might have been innovated in the supergroup of opisthokonts as well as structural diversification in the axonemes at the base of bikonts and opisthokonts. Further phylogenetic or structural evidence is necessary to conclude the evolutional and reproductive significance of calaxin.
References
Blum JJ, Hayes A, Jamieson GA Jr, Vanaman TC (1980) Calmodulin confers calcium sensitivity on ciliary dynein ATPase. J Cell Biol 87:386–397
Brokaw CJ (1979) Calcium-induced asymmetrical beating of triton-demembranated sea urchin sperm flagella. J Cell Biol 82:401–411
Brokaw CJ, Nagayama SM (1985) Modulation of the asymmetry of sea urchin sperm flagellar bending by calmodulin. J Cell Biol 100:1875–1883
Burgoyne RD (2004) The neuronal calcium-sensor proteins. Biochim Biophys Acta 1742:59–68
Burgoyne RD, Weiss JL (2001) The neural calcium-sensor family of Ca2+-binding proteins. Biochem J 353:1–12
Casey DM, Inaba K, Pazour GJ, Takada S, Wakabayashi K, Wilkerson CG, Kamiya R, Witman GB (2003) DC3, the 21-kDa subunit of the outer dynein arm-docking complex (ODA-DC), is a novel EF-hand protein important for assembly of both the outer arm and the ODA-DC. Mol Biol Cell 14:3650–3663
Gibbons BH, Gibbons IR (1980) Ca2+-induced quiescence in reactivated sea urchin sperm. J Cell Biol 84:13–27
Gitelman SE, Witman GB (1980) Purification of calmodulin from Chlamydomonas: calmodulin occurs in cell bodies and flagella. J Cell Biol 8:764–770
Hozumi A, Satouh Y, Makino Y, Toda T, Ide H, Ogawa K, King SM, Inaba K (2006) Molecular characterization of Ciona sperm outer arm dynein reveals multiple components related to outer arm docking complex protein 2. Cell Motil Cytoskeleton 63:591–603
Inaba K (2007) Molecular basis of sperm flagellar axonemes: structural and evolutionary aspects. Ann N Y Acad Sci 1101:506–526
Jamieson GA Jr, Vanaman TC, Blum JJ (1979) Presence of calmodulin in Tetrahymena. Proc Natl Acad Sci USA 76:6471–6475
Kambara Y, Shiba K, Yoshida M, Sato C, Kitajima K, Shingyoji C (2011) Mechanism regulating Ca2+-dependent mechanosensory behaviour in sea urchin spermatozoa. Cell Struct Funct 36:69–82
Kamiya R, Okamoto M (1985) A mutant of Chlamydomonas reinhardtii that lacks the flagellar outer dynein arm but can swim. J Cell Sci 74:181–191
Kamiya R, Witman GB (1984) Submicromolar levels of calcium control the balance of beating between the two flagella in demembranated models of Chlamydomonas. J Cell Biol 98:97–107
King SM, Patel-King RS (1995) Identification of a Ca2+-binding light chain within Chlamydomonas outer arm dynein. J Cell Sci 108:3757–3764
Lindemann CB, Goltz JS (1988) Calcium regulation of flagellar curvature and swimming pattern in triton X-100-extracted rat sperm. Cell Motil Cytoskeleton 10:420–431
Lindemann CB, Gardner TK, Westbrook E, Kanous KS (1991) The calcium-induced curvature reversal of rat sperm is potentiated by cAMP and inhibited by anti-calmodulin. Cell Motil Cytoskeleton 20:316–324
Mitchell DR, Rosenbaum JL (1985) A motile Chlamydomonas flagellar mutant that lacks outer dynein arms. J Cell Biol 100:1228–1234
Mizuno K, Padma P, Konno A, Satouh Y, Ogawa K, Inaba K (2009) A novel neuronal calcium sensor family protein, calaxin, is a potential Ca2+-dependent regulator for the outer arm dynein of metazoan cilia and flagella. Biol Cell 101:91–103
Mizuno K, Shiba K, Okai M, Takahashi Y, Shitaka Y, Oiwa K, Tanokura M, Inaba K (2012) Calaxin drives sperm chemotaxis by Ca2+-mediated direct modulation of a dynein motor. Proc Natl Acad Sci USA 109:20497–20502
Naitoh Y, Kaneko H (1972) Reactivated triton-extracted models of Paramecium: modification of ciliary movement by calcium ions. Science 176:523–524
Nakano I, Kobayashi T, Yoshimura M, Shingyoji C (2003) Central-pair-linked regulation of microtubule sliding by calcium in flagellar axonemes. J Cell Sci 116:1627–1636
Okada M, Takezawa D, Tachibanaki S, Kawamura S, Tokumitsu H, Kobayashi R (2003) Neuronal calcium sensor proteins are direct targets of the insulinotropic agent repaglinide. Biochem J 375:87–97
Padma P, Satouh Y, Wakabayashi K, Hozumi A, Ushimaru Y, Kamiya R, Inaba K (2003) Identification of a novel leucine-rich repeat protein as a component of flagellar radial spoke in the ascidian Ciona intestinalis. Mol Biol Cell 14:774–785
Piperno G, Mead K, Shestak W (1992) The inner dynein arms I2 interact with a “dynein regulatory complex” in Chlamydomonas flagella. J Cell Biol 118:1455–1463
Saito T, Shiba K, Inaba K, Yamada L, Sawada H (2012) Self-incompatibility response induced by calcium increase in sperm of the ascidian Ciona intestinalis. Proc Natl Acad Sci USA 109:4158–4162
Sakato M, King SM (2003) Calcium regulates ATP-sensitive microtubule binding by Chlamydomonas outer arm dynein. J Biol Chem 278:43571–43579
Sakato M, Sakakibara H, King SM (2007) Chlamydomonas outer arm dynein alters conformation in response to Ca2+. Mol Biol Cell 18:3620–3634
Sale WS (1986) The axonemal axis and Ca2+-induced asymmetry of active microtubule sliding in sea urchin sperm tails. J Cell Biol 102:2042–2052
Shiba K, Baba SA, Inoue T, Yoshida M (2008) Ca2+ bursts occur around a local minimal concentration of attractant and trigger sperm chemotactic response. Proc Natl Acad Sci USA 105:19312–19317
Shingyoji C, Takahashi K (1995) Flagellar quiescence response in sea urchin sperm induced by electric stimulation. Cell Motil Cytoskeleton 31:59–65
Smith EF (2002) Regulation of flagellar dynein by calcium and a role for an axonemal calmodulin and calmodulin dependent kinase. Mol Biol Cell 13:3303–3313
Smith EF, Yang P (2004) The radial spokes and central apparatus: mechano-chemical transducers that regulate flagellar motility. Cell Motil Cytoskeleton 57:8–17
Tash JS, Means AR (1983) Cyclic adenosine 3',5' monophosphate, calcium and protein phosphorylation in flagellar motility. Biol Reprod 28:75–104
Tash JS, Krinks M, Patel J, Means RL, Klee CB, Means AR (1988) Identification, characterization, and functional correlation of calmodulin-dependent protein phosphatase in sperm. J Cell Biol 106:1625–1633
Wakabayashi K, Yagi T, Kamiya R (1997) Ca2+-dependent waveform conversion in the flagellar axoneme of Chlamydomonas mutants lacking the central-pair/radial spoke system. Cell Motil Cytoskeleton 38:22–28
Wakabayashi K, King SM (2006) Modulation of Chlamydomonas reinhardtii flagellar motility by redox poise. J Cell Biol 173:743–754
Witman GB (1993) Chlamydomonas phototaxis. Trends Cell Biol 3:403–408
Acknowledgments
We thank Y. Shikata, K. Oiwa, H. Sakakibara, H. Kojima, S.A. Baba, O. Kutomi, K. Hirose, M. Okai, Y. Takahashi, M. Tanokura, K. Seto, and Y. Degawa for useful advice in the present study. We are grateful to all staff members of the Education and Research Center of Marine Bio-Resources, Tohoku University, and to the National Bio Resource Project (NBRP) for supplying C. intestinalis. This work was supported in part by a grant from MEXT (Ministry of Education, Culture, Sports, Science and Technology), Japan, and by JST-BIRD (Japan Science and Technology Agency-Institute for Bioinformatics Research and Development), Japan, to K.I.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
This chapter is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
Copyright information
© 2014 The Author(s)
About this paper
Cite this paper
Inaba, K., Mizuno, K., Shiba, K. (2014). Structure, Function, and Phylogenetic Consideration of Calaxin. In: Sawada, H., Inoue, N., Iwano, M. (eds) Sexual Reproduction in Animals and Plants. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54589-7_5
Download citation
DOI: https://doi.org/10.1007/978-4-431-54589-7_5
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54588-0
Online ISBN: 978-4-431-54589-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)