Abstract
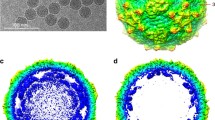
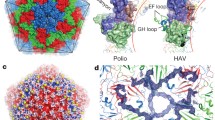
Picornaviruses are small, nonenveloped, icosahedral viruses. The mature virions are approximately 300 Å in diameter and the interior of the capsid shell is packed with single-stranded plus sense RNA (Mw ∼ 8×106 Daltons) (Rueckert 1990). The virus capsid is composed of 60 protomers, each consisting of one copy of the viral proteins VP1, VP2, VP3 and VP4 arranged in a T= 1 (Pseudo T = 3) icosahedral lattice (CASPAR and KLUG 1962) (Fig. 1). Atomic resolution structures have been determined for all Picornaviridae genera (Hogle et al. 1985; Rossmann et al. 1985; Luo et al. 1987; Acharya et al. 1989) with the exception of the hepatoviruses. Their structures demonstrate conservation of the eight-stranded antiparallel β-sandwich of VP1, VP2 and VP3 while VP4, which is completely interior, is not well defined in many of the structures. The regions of most structural dissimilarity for VP1, VP2 and VP3 occurs at the NH2- and COOH-terminals and the loops that connect the β-strands. Many of the differences at the COOH-terminals and β-strand loops present themselves on the surface of the virion and give each virus its unique surface topology and hence define how these viruses interact with cells and mediate early steps in the viral life cycle including receptor recognition and viral uncoating.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Achaiya R, Fry E, Stuart D, Fox G, Rowlands D, Brown F (1989) The three-dimensional structure of foot-and-mouth disease virus at 2.9A resolution. Nature 337:709–716
Ansardi DC, Porter DC, Morrow CD (1992) Myristylation of poliovirus capsid precursor PI is required for assembly of subviral particles. J Virol 66:4556—4563
Badger J, Minor I, Oliveira MA, Smith TJ, Rossmann MG (1989) Structural analysis of antiviral agents that interact with the capsid of human rhinoviruses. Proteins 6:1–19
Bergelson JM, Mohanty JG, Crowell RL, St. John N, Lublin DM, Finberg RW (1995) Coxsackievirus B3 adapted to growth in RD cells binds to decay-accelerating factor (CD55). J Virol 69:1903–1906
Caggana M, Chan P, Ramsingh A (1993) Identification of a single amino acid residue in the capsid protein VP1 of coxsackievirus B4 that determines the virulent phenotype. J Virol 67:4797–4803
Caspar DLD, Klug A (1962) Physical principles in the construction of regular viruses. Cold Springs Harbor Symp Quant Biol 27:1–2
Chapman MS, Rossmann MG (1993) Comparison of surface properties of picornaviruses: strategies for hiding the receptor site from immune surveillance. Virology 195:745–756
Chelvanayagam G, Heringa J, Argos P (1992) Anatomy and evolution of proteins displaying the viral capsid jellyroll topology. J Mol Biol 228:220–242
Chow M, Newman JFE, Filman D, Hogle JM, Rowlands DJ, Brown F (1987) Myristylation of picornavirus capsid protein VP4 and its structural significance. Nature 327:482–486
Colonno RJ, Condra JH, Mizutani S, Callahan PL, Davies M, Murcko MA (1988) Evidence for the direct involvement of the rhinovirus canyon in receptor binding. Proc Natl Acad Sci 85:5449–5453
Colston E, Racaniello VR (1994) Soluble receptor-resistant poliovirus mutants identify surface and internal capsid residues that control interaction with the cell receptor. EMBO J 13:5855–5862
Filman DJ, Syed R, Chow M, Macadam AJ, Minor PD, Hogle J (1989) Structural factors that control conformational transitions and serotype specificity in type 3 poliovirus. Embo J 8:1567–1579
Fox MP, Otto MJ, McKinlay MA (1986) The prevention of rhinovirus and poliovirus uncoating by WIN 51711: a new antiviral drug. Antimicrob Agents Chemother 30:110–116
Gauntt CJ, Trousdale MD, LaBadie DRL, Paque RE, Nealon T (1979) Properties of coxsackievirus B3 variants which are amyocarditic or myocarditic for mice. J Med Virol 3:207–220
Giranda VL, Heinz BA, Oliveira MA, Minor I, Kim KH, Kolatkar PR, Rossmann MG, Rueckert, RR (1992) Acid-induced structural changes in human rhinovirus 14: possible role in uncoating. Proc Natl Acad Sci USA 89:10213–10217
Grant RA, Hiremath CN, Filman DF, Syed R, Andries K, Hogle JM (1994) Structures of poliovirus complexes with anti-viral drugs: Implications for viral stability and drug design. Curr Biol 4:784–797
Heinz BA, Shepard DA, Rueckert RR (1990) Escape mutant analysis of a drug-binding site can be used to map functions in the rhinovirus capsid. In: Laver WGAir GM (eds) Use of x-ray crystallography in the design of antiviral agents. Academic, New York, pp 173–186
Hogle JM, Chow M, Filman DJ (1985) Three-dimensional structure of poliovirus at 2.9A resolution. Science 229:1358–1365
Hsu KL, Paglini S, Alstein B, Crowell RL (1990) Identification of a second cellular receptor for a coxsackievirus B3 variant, CVB3-RD. In: Brinton MAHeinz FX (eds) New aspects of positive strand RNA viruses. American Society for Microbiology, Washington, DC, pp 271–277
Kim KH, Willingmann P, Gong ZX, Kremer MJ, Chapman MS, Minor I, Oliveira MA, Rossmann MG, Andries K, Diana GD, Dutko FJ, McKinlay MA, Pevear DC (1993) A comparison of the anti-rhinoviral drug binding pocket in HRV14 and HRV1A.J Mol Biol 230:206–227
Kim S, Smith TJ, Chapman MS, Rossmann MG, Pevear DC, Dutko FJ, Felock PJ, Diana GD, McKinlay MA (1989) The crystal structure of human rhinovirus serotype 1A (HRV1A). J Mol Biol 210:91–111
Lindberg MA, Crowell RL, Zell R, Kandolf R, Pettersson U (1992). Mapping of the RD phenotype of the Nancy strain of coxsackievirus B3. Virus Research 24:187–196
Luo M, Vriend G, Kamer G, Minor I, Arnold E, Rossmann MG, Boege U, Scraba DG, Duke GM, Palmenberg A (1987) The atomic structure of Mengo virus at 3.0A resolution. Science 235:182–191
Mapoles JE, Krah DL, Crowell RL (1985) Purification of a HeLa cell receptor protein for the group B coxsackieviruses. J Virol 55:560–566
Melnick JL (1990) Enteroviruses: polioviruses, coxsackieviruses, echoviruses and newer enteroviruses. In: Fields BKnipe D (eds) Virology, 2nd edn. Raven, New York, 1:549–605
Minor PD, Ferguson M, Evans DM, Almond JW, Icenogle JP (1986) Antigenic structure of polioviruses of serotypes 1, 2 and 3. J Gen Virol 67:1283–1291
Moscufo N, Chow M (1992) Myristate-protein interactions in poliovirus: Interactions of VP4 threonine 28 contribute to the structural conformation of assembly intermediates and the stability of assembled virions. J Virol 66:6849–6857
Muckelbauer JK, Kremer M, Minor I, Diana G, Dutko FJ, Groarke J, Pevear DC, Rossmann MG (1995) The structure of coxsackievirus B3 at 3.5A resolution. Structure 15:653–667
Muckelbauer JK, Kremer M, Minor I, Tong L, Zlotnick A, Johnson JE, Rossmann MG (1996) The structure determination of coxsackievirus B3 to 3.5A resolution. Acta Crystallogr D51:871–887
Murray MG, Bradley J, Yang XF, Wimmer E, Moss EG, Racaniello VR (1988) Poliovirus host range is determined by a short amino acid sequence in neutralizing antigenic site 1. Science 241:213–215
Oliveira MA, Zhao R, Lee W-M, Kremer MJ, Minor I, Rueckert RR, Diana GD, Pevear DC, Dutko FJ, McKinlay MA, Rossmann MG (1993). The structure of human rhinovirus 16. Structure 1:51–68
Olson NH, Kolatkar PR, Oliveira MA, Cheng RH, Greve JM, McClelland A, Baker TS, Rossmann MG (1993) Structure of a human rhinovirus complexed with its receptor molecule. Proc Natl Acad Sci USA 90:507–511
Page GS, Mosser AG, Hogle JM, Filman DJ, Rueckert RR, Chow M (1988) Three-dimensional structure of poliovirus serotype 1 neutralizing determinants. J Virol 63:1781–1794
Palmenberg AC (1989) Sequence alignments of picornaviral capsid proteins. In: Semler BLEhrenfeld E (eds) Molecular aspects of picornavirus infection and detection. American Society for Microbiology, Washington, DC, pp 211–241
Pevear DC, Fancher MJ, Felock PJ, Rossmann MG, Miller MS, Diana G, Treasurywala AM, McKinlay MA, Dutko FJ (1989) Conformational changes in the floor of the human rhinovirus canyon blocks adsorption to HeLa cell receptors. J Virol 63:2202–2207
Reagan KJ, Goldberg G, Crowell RL (1984). Altered receptor specificity of coxsackievirus B3 after growth in rhabdomyosarcoma cells. J Virol 49:635–640
Reimann BY, Zell R, Kandolf R (1991) Mapping of a neutralizing antigenic site of coxsackievirus B4 by construction of an antigen chimera. J Virol 65:3475–3480
Rossmann MG (1989) The canyon hypothesis. J Biol Chem 264:14587–14590
Rossmann MG (1994) Viral cell recognition and entry. Prot Sci 3:1712–1725
Rossmann MG, Palmenberg AC (1988) Conservation of the putative receptor attachment site in picornaviruses. Virology 164:373–382
Rossmann MG, Arnold E, Erickson JW, Frankenberger EA, Griffith JP, Hecht H-J, Johnson JE, Kamer G, Luo M, Mosser AG, Rueckert RR, Sherry B, Vriend G (1985) Structure of a human common cold virus and functional relationship to other picornaviruses. Nature 317: 145–156
Rueckert RR (1990) Picornaviridae and their replication. In: Fields B, Knipe D (eds) Virology, 2nd edn, Raven, New York, 1:507–548
Sherry B, Rueckert RR (1985) Evidence for at least two dominant neutralization antigens on human rhinovirus 14. J Virol 53:137–143
Sherry B, Mosser AG, Colonno RJ, Rueckert RR (1986) Use of monoclonal antibodies to identify four neutralization immunogens on a common cold picornavirus, human rhinovirus 14. JVirol 57:246–257
Smith TJ, Kremer MJ, Luo M, Vriend G, Arnold E, Kamer G, Rossmann MG, McKinlay MA, Diana GD, Otto MJ (1986) The site of attachment in human rhinovirus 14 for antiviral agents that inhibit uncoating. Science 233:1286–1293
Zhang A, Nanni RG, Li T, Ferstandig Arnold G, Oren DA, Jacobo-Molina A, Williams RL, Kamer G, Rubenstein DA, Li Y, Rozhon E, Cox S, Buontempo P, O’Connell J, Schwartz J, Miller G, Bauer B, Versace R, Pinto P, Ganguly A, Girijavallabhan V, Arnold E (1993) Structure determination of antiviral compound SCH 38057 complexed with human rhinovirus 14. J Mol Biol 230:857–867
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1997 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Muckelbauer, J.K., Rossmann, M.G. (1997). The Structure of Coxsackievirus B3. In: Tracy, S., Chapman, N.M., Mahy, B.W.J. (eds) The Coxsackie B Viruses. Current Topics in Microbiology and Immunology, vol 223. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-60687-8_9
Download citation
DOI: https://doi.org/10.1007/978-3-642-60687-8_9
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-64507-5
Online ISBN: 978-3-642-60687-8
eBook Packages: Springer Book Archive