Abstract
The collaboration of blood transfusion service in the management of severely combat-injured individuals has proved to be an essential factor for the successful treatment of these patients. While the operating and anesthesiology teams are engaged in maintaining the vital signs and controlling blood loss of the injured, the transfusion service representatives follow the information on the amount of blood products given and the latest laboratory tests, as well as provide consultations regarding further blood component requirements on the basis of data obtained. A major effort of the treating team should be aimed at diagnosis and correction of coagulopathy, acidosis, and hypothermia. For the massively bleeding combat trauma injured, which can amount to as high as 8% of all trauma patients, a generous use of plasma at a one-to-one ratio with packed cells, along with the early addition of platelets and cryoprecipitates, should be considered. Early point-of-care thromboelastography is helpful for identification of coagulopathies. The use of a preset massive transfusion protocol is beneficial; however, it should be tailored according to the patient’s actual needs, depending on the type of injury and the individual’s general condition.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Injury Severity Score
- Fresh Freeze Plasma
- Adult Respiratory Distress Syndrome
- Massive Transfusion
- Recombinant Factor VIIa
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
6.1 History of Blood Transfusion for Combat Injuries
The first blood transfusion was given to patients by James Blundell who initiated the transfusion of human blood to ten patients in 1818; five survived, four had a postpartum hemorrhage, and the fifth was a boy who bled after amputation. Lieutenant Oswald Robertson of the US Army Medical Officers’ Reserve Corps initiated the use of citrated blood for military conflict victims in the battlefield during World War I (1917), following the discovery by Peyton Rous in 1915 that citrate added to collected blood could prevent coagulation. Major L. Bruce Robertson reported the use of citrated blood in a casualty clearing station, with the blood being drawn from volunteers in the field and transfused to wounded individuals who had significant blood loss, were in a state of shock and, therefore, were a poor operative risk [33]. A blood service providing stored citrated blood became available during the Spanish Civil War, both in Madrid, where Norman Bethune, leading the Canadian medical team in 1936–1937, founded a mobile blood transfusion service, and in Barcelona, where similar corps were organized by Duran F Jorda. It developed into a fully established service in the London Blitz. During World War II, acid citrate glucose solution began to be added in a small volume of 70 cc to 450 ml of blood donation. While American troops used lyophilized plasma until 1945 for transfusions on the battlefield, British Armed Forces employed citrated whole blood. During the Korean War, the most common blood product used was whole blood (500 ml bottles) and sometimes 30 pints of blood or even more were transfused to seriously wounded individuals in forward field hospitals during the first few hours after injury. Most of the blood was collected in the United States and transported to Korea by air. The O(Rh+) D positive whole blood was used for transfusion, although it was not matched prior to the procedure [6]. Stored whole blood (500 ml) continued to be used during the Vietnam War. At that time, plastic bags and tubing became available for blood collection and storage. Until April 1965, only O whole blood units were shipped from Japan but, by the end of 1965, all the blood shipped was ABO and matched ABO units could be administered [18]. Fresh frozen plasma (FFP) was introduced in October 1968 for casualties with coagulopathy, and 5,965 units of FFP were reported to have been administered by December 1972. The ratio of FFP to stored whole blood administered in that arena was 1:172 units [25].
Pfefferman et al. [30] described the experience of a forward evacuation hospital on the Sinai front during the Israeli-Arab war in October 1973 and reported a 23% mortality rate among 51 combat injured patients evacuated to the hospital and operated immediately upon admittance. A mean of 15 units of blood were transfused to these individuals. The first 8–10 units of blood given to each casualty were taken from the stored blood supply, which was less than 24 h old. When more blood was needed, it was drawn from donors into citrated bottles and immediately transfused to patients. Of the 12 injured who succumbed, three died of uncontrolled major vessel bleeding, two due to neurological causes and seven due to sepsis. All blood transfused was fresh whole blood by today’s criteria, containing both fresh plasma with active coagulation factors and active platelets. The use of this modality was possible because of the short flight time from the blood donation centers (about 3 h) and a large number of volunteers donating blood at that time.
6.2 Currently Available Blood Products
Presently, in most developed countries, the use of whole blood in the management of severely combat injured individuals is considered obsolete for several reasons. Whole blood which includes both donor red blood cells and plasma is kept at 1–6°C. The product is known to lose activity of factors VIII and V within the first 24–48 h following donation. Platelets kept at low temperatures become dysfunctional within hours and there is no hemostatic benefit for this product. For these reasons and more, whole blood is currently separated into several components shortly after donation (Table 6.1). Packed red blood cells (PRBC) are kept in storage at 1–6°C for up to 35 or 42 days depending on the anticoagulant/preservation solution. Fresh frozen plasma is frozen shortly after collection and is stored at −18°C for a year. Cryoprecipitate contains mainly fibrinogen, factor VIII, von Willebrand factor and factor XIII, and is stored at −18°C or lower for a year. This product is not available in many European countries where coagulation factor concentrates, prepared from large pools of donor plasma and undergoing solvent/detergent treatment to increase safety, are in use.
Three types of platelet concentrates are currently available. The first is a random donor platelet concentrate prepared from individual units kept in special breathable bags and given in four to eight pooled units. This product is very sensitive to bacterial contamination and is kept for up to 5 days on a swirling tray (constant gentle agitation) in the blood bank. The second product, now transfused to about 90% of US patients, is single donor platelets prepared by apheresis, kept at 20–24°C for up to 5 days on a swirling tray, and containing 3 × 1011 platelets.
Recently, frozen platelets kept at −80°C, thawed and washed to remove preservation solution, were used in Iraq and Afghanistan by the Netherlands Armed Forces transfusion service stationed there [19]. These single donor platelets are frozen in DMSO and may be kept up to 2 years at −80°C. The processing time of thawing and resuspension is about 30–40 min. Once the product is thawed and washed it should be administered within 6 h. An in vivo viability assay of cryo-preserved and thawed platelets infused to a SCID mice model revealed a 4-h survival of 40% versus 80% for fresh platelets and estimated half-lives of 2.5 versus 7 h, respectively [42].
The use of fresh whole blood for massively bleeding trauma patients was considered as having an almost miraculous effect on bleeding patients, but was first rejected by blood bankers and transfusion specialists due to serious safety issues [36]. The use of blood collected less than 24 h prior to transfusion and kept at 25°C provides platelet viability, retaining most of the coagulation factors, with no damage to red blood cells due to the storage effect, and increased immediate oxygen carrying capacity compared to stored blood. On the other hand, this is a very wasteful way to handle blood inventory, given that a blood unit loses its advantages after 24 h of storage and needs to be discarded since it was kept in a nonrefrigerated environment for 24 h. The period of time between blood donation and its administration as fresh whole blood is too short to perform the current state-of-the-art virology tests, including testing for viral nucleic acids; therefore, this product is relatively unsafe and should be reserved only for extreme conditions of acute shortage of blood products supply.
Spinella et al. retrospectively examined 2,831 samples from fresh whole blood donor units transfused in Iraq and Afghanistan over 3 years and found five positive recombinant Immunoblot assays – three for hepatitis C and two for HTLV1 virus [37]. Another hazard is the possibility of transfusion-associated graft-versus-host disease due to infusion of 108 viable white blood cells that have not been irradiated. A similar cohort of patients with combat-related trauma who were given at least one unit of blood was retrospectively assessed. Resuscitation with combined fresh whole blood revealed a 30-day survival of 95%, while the use of stored components revealed survival of 82% [36]. It should be kept in mind that these are retrospective studies where the groups receiving fresh whole blood were given additional VIIa at a higher rate than the group receiving blood components. Another worrisome issue is the fact that the mean age of blood transfused to combat wounded patients was 33 days [37], which is very close to the expiration date, while the mean age of blood usually given to civilian trauma victims is less than 15 days. Perkins et al. retrospectively evaluated the use of fresh whole blood for massively bleeding trauma patients compared to component therapy that included apheresis platelets. Among a cohort of 8,618 patients treated in Iraq from 1/2004 to 12/2006, 128 were treated with fresh whole blood. There was no survival benefit for those treated with fresh whole blood and adult respiratory distress syndrome (ARDS) was more prevalent in this group of patients. Time to supply of fresh whole blood was 3–4 h, and for apheresis platelets was 2.4 h [29].
The current Joint Theater Trauma System Clinical Practice Guidelines recommend a “last in, first out” policy, meaning an attempt to give the youngest units of blood available for patients in need of massive transfusion. The recommendation is to give PRBC less than 14 days old.
The Netherlands Military Transfusion Service developed a system to minimize the waste of blood products and ensure their stable supply. Packed red blood cells were frozen in 40% glycerol where they can be kept for up to 10 years at −80°C. Deep frozen plasma could be kept for 7 years at −80°C and platelets for up to 2 years. The products are thawed and washed using automated closed system cell processor (ACP 215). Packed red blood cells may be kept at 4°C for up to 14 days, and platelets for up to 6 h following thawing, washing, and resuspension in thawed plasma [19]. This system requires stable power supply although backup system of liquid CO2 is installed.
6.3 Role of Coagulopathy in the Outcome of Trauma Patients
Coagulopathy of trauma is one of the main contributors to mortality in patients with massive bleeding. It has been considered a grave prognostic sign in trauma patients hospitalized in intensive care units [9]. Trauma-induced coagulopathy can develop in 24% of patients independent of hypothermia or acidosis [3, 11]. Massive bleeding affects coagulation in several ways, including dilution or consumption of coagulation factors, cell shock generating cellular acidosis, tissue injury that results in further consumption of coagulation factors, and iatrogenic causes such as cold fluids infusion that bring about hypothermia. All these factors led Cosgriff et al. to develop “the bloody vicious cycle model” of hemodilution, hypothermia, and acidosis, all contributing to coagulopathy [5]. Additional factors, such as platelet dysfunction due to anemia, increased fibrinolysis, reduced platelet count, and coagulation factor deterioration, may also contribute to trauma-induced coagulopathy [10]. In several reported studies, coagulopathy was found to increase mortality in patients with an injury severity score (ISS) >15 [3, 20]. A 38% prevalence of acute coagulopathy was revealed using point-of-care devices in transfused combat casualties upon their arrival to the 31st Combat Support Hospital in Iraq. The mortality rate of patients suffering from coagulopathy was 24% compared with 4% in those not presenting with coagulopathy. Coagulopathy and acidosis had mortality odd ratios of 5.3 and 6.9, respectively [27].
In a small cohort of young soldiers evacuated from the battlefield in 2006 who required massive transfusions as part of their management, even those who did not have coagulopathy on arrival, as happened in about 24% of cases, developed it during the first 2 h post-admission. In 21 military trauma victims with massive bleeding, a median INR (prothrombin time international normalized ratio) of 1.26 (range, 1.12–2.2) was recorded at admission to hospital with a median value of 1.55 (range, 1.24–2.31) at 2 h after admission [7]. These results suggest the presence of coagulopathy as long as bleeding is not fully controlled. Since coagulopathy of trauma is known to be a major poor prognostic factor, an attempt has been made in recent years to preempt its development by the early use of plasma and platelets in massively bleeding trauma victims.
6.4 Preemptive Therapy of Coagulopathy
Based on a computerized model, Hirshberg et al. [12] suggested that massively bleeding patients should be treated with a 2:3 ratio of FFP to packed red blood cells (PRBC) to adequately supply coagulation factors. Ho et al. [13] compared various strategies of fresh frozen plasma transfusion for the prevention or correction of dilution coagulopathy. Once coagulopathy developed, a ratio of 1 or 1.5 FFP to every unit of PRBC had to be transfused in order to correct coagulopathy. Borgman et al. [2] reported a retrospective study of 246 patients treated in a combat support hospital who received massive transfusion of 10 units of blood or more. The patients were stratified by a median ratio of FFP:PRBC. A low FFP to PRBC ratio was defined as 1:8, medium as 1:2.5, and high as 1:1.4. The median ISS was 18 and mortality was 65% for the low ratio group, 34% for the medium ratio group, and 19% for the high ratio group. In these groups, mortality due to hemorrhage was 92%, 78%, and 37%, respectively. The intuitive message from this report was that a 1:1 FFP to PRBC ratio may save many lives in combat-induced trauma, and such a policy was widely adopted. We still need to keep in mind that this was a retrospective study, where the effect of a learning curve on the treatment of patients is possible. Moreover, since the protocol at the beginning of the study did not imply initiating therapy with a 1:1 FFP:PRBC ratio and plasma was given only after several units of PRBC, a biased population could be created. The median times to death in the low, medium, and high ratio groups were 2, 4, and 38 h, respectively. It is also possible that the patients who received a low FFP to PRBC ratio did not survive long enough to get plasma. A patient who needs 10 units of blood within 90 min may not be comparable to the one who requires 10 units of blood over a 24-h period.
Currently adopted 1:1 FFP to PC ratio for penetrating wound injuries was examined also in the civilian milieu where blunt injury is more common. A prospective multicenter study of the National Institute of General Medical Sciences performed during the years 2003–2007 had the following eligibility criteria: patients with a blunt mechanism of injury, pre-hospital or emergency department systolic blood pressure of <90 mm Hg or an elevated base deficit (≥6 meq/L), blood transfusion requirement within the first 12 h, and any injury with an abbreviated injury score of (AIS) ≥2, allowing the exclusion of patients with isolated traumatic brain injury. Among 1,036 patients with blunt injury enrolled in the study, the overall mortality was 33.5% and the overall complication rates of multi-organ failure, incidence of nosocomial infection, and adult respiratory distress syndrome were 56.4%, 46.5%, and 19.6%, respectively. When only patients requiring ≥8 of PRBC during the first 12 h were analyzed (n = 415), the following results were obtained. Comparison of patients receiving a high ratio of FFP:PRBC (1:2; 1:3–4) with those receiving a low FFP:PRBC (≤1:5) demonstrated the high ratio to be a significant independent predictor of survival (mortality HR0.38, p = 0.002) [35]. Twenty-four hours mortality for the high ratio group was 3.95% compared with 12.8% for the low ratio cohort (p = 0.012). The group of patients who received a higher FFP:PRBC ratio required a lower dose of PRBC, but received a greater amount of plasma and cryoprecipitate. There was no significant difference in overall survival [35]. The same cohort of patients, including all those who had blunt trauma, was analyzed for multi-organ failure and ARDS, excluding the patients who succumbed during the first 24 h. Of the entire study population (n = 1175), 65%, 41%, and 28%, respectively, of patients received FFP, platelets, and cryoprecipitate. In regard to every unit given, FFP was independently associated with a 2.1% and 2.5% increased risk of multi-organ failure (MOF) and ARDS, respectively. Cryoprecipitate was associated with a 4.4% decrease of MOF, while there was no association between platelet transfusion and any of the outcomes examined. When early deaths were also included in the assessment model, FFP was found to be associated with a 2.9% decrease in the risk of mortality per unit transfused [41]. The data suggest that the early use of FFP, cryoprecipitate, and platelets is beneficial to overcome coagulopathy of trauma and should be initiated early in massively transfused injured. However, for patients who require a lesser amount of blood and do not have coagulopathy, both benefits and risks should be assessed.
6.5 Northern Israel’s Experience in Handling Massively Bleeding Trauma Patients
On July 12, 2006, two Israeli soldiers were kidnapped and three others were killed by Hizbullah gunmen within Israeli territory. This incident led to heavy bombing of Lebanon and massive rocket attacks on northern Israel, home to 1.5 million people. A total of 3,970 rockets were fired in that area, destroying thousands of buildings and taking the lives of 39 civilians and 117 soldiers. During the 33 days of warfare, the wounded were evacuated to four regional hospitals and one level 1 trauma center. Transfusion management of the injured in this conflict has been reported previously [8].
Modern armament is known to cause multidimensional injuries. Their efficient treatment frequently necessitates transfusion of blood products or massive blood transfusions. The requirement for blood products for civilian trauma patients in the 2006 conflict was 6% of the trauma victims, similar to the number of patients (8%) who received PRBC in the urban trauma center in Baltimore [4]. In comparison, the blood usage in 6,533 injured civilians evacuated from the terrorist attack scene to nearby hospitals in the years 2000–2005 was 23%. Such a high rate of blood use in the latter case reflects a different origin and extension of injury resulting from shrapnel and metal bolts embedded in the bombs [34]. Blood requirements of wounded soldiers admitted to the Rambam Trauma Center during the 2006 conflict was 16%, twice as high as that reported by an urban trauma center in the US. Forty-one percent of admitted soldiers requiring blood had massive transfusions, compared with 13%, 24%, and 30% reported by Huber-Wagner et al. [14], Malone et al. [21], and Como et al. [4], respectively, using urban trauma findings. US Army transfusion data, obtained during 10 months of activities in Iraq (beginning with March 2003), showed that massive transfusions were given to 60 of 281 injured patients (21%) [16].
The high rate of massive transfusions may reflect selected airlift evacuation to the level 1 trauma center. Another worrisome finding is the mean ISS of 19.2, ranging from 6 to 75, compared to the mean ISS of 24.5, 22, and 21 reported in the urban trauma cohorts [4, 14, 21]. The relatively low ISS of patients receiving massive transfusions observed in the Rambam Trauma Center may reflect an excessive blood loss during prolonged evacuation, a median of 2 h for injured with ISS ≥ 16 and 3.3 h for ISS <16, compared to the 72 min reported in the German Trauma Society cohort [14]. It could also be associated with the devastating effect of modern anti-tank missiles and other armament, since most soldiers had penetrating wounds, while the vast majority of reported civilian patients had blunt traumas. These data show that the number of victims requiring blood transfusion is directly related to the kind of armament used at the conflict arena (improvised explosive devices, mortars, anti-tank missiles, ground-to-ground rockets, low velocity guns, mines, etc).
6.5.1 The Threshold of Blood Product Transfusion
Indications for the use of blood products in patients with combat trauma are similar to those for civilian trauma patients. Sufficient oxygen supply to tissues is essential for acidosis prevention and, as long as full control of bleeding is not achieved, a hematocrit of 30 should be maintained to provide oxygenation for vital organs and improve delivery of platelets to vessel walls, where they are needed for bleeding control. Since coagulopathy is a major detrimental factor for survival, observed in about one quarter of patients, preemptive therapy should be initiated immediately in patients with predictive massive bleeding. It is essential to draw blood for type, screen, and cross-match immediately on admission to the trauma bay. Proper temporary labeling of unidentified unconscious injured at that point is crucial, since a mislabeled blood sample for type and cross could end with mistransfusion and death (Fig. 6.1 a and b). The blood bank should be notified if a patient received blood on the way to the trauma bay, since mixed RBC populations could be observed by the lab technician, if a patient with non-O type received O type blood. Simultaneously, blood samples for coagulation studies, such as prothrombin time (PT), partial thromboplastin time (PTT), and fibrinogen level, should be sent to the lab marked as “immediate priority”. Transfused non-cross matched blood should be O D+ for a male patient and O D− for a female patient, if “non” typed blood is required. Usually, immediate blood typing can be performed within 5 min of arrival of a blood sample to the lab, and a full cross-matching and screening takes about 30 min and can be completed within this time frame.
Label samples. Upon admission to the emergency department (ED), each patient receives a sequential four-digit case number, then a personal identification number (ID), and other details are recorded whenever possible. Both case and ID numbers appear on each patient’s label. Application of these two numbers reduces the possibility of misidentification in mass casualty events. Although a four-digit bold number is easy to verify, the computer system requires an eight-digit ID number, which is equal to the number of digits in the state ID. Therefore, a temporary eight-digit ID number is given to unidentified patients as well. Following specific identification of a patient, the temporary ID number is changed for the state number, and personal details, such as name and age, are added to the labels of these individuals. The case number issued at admission is not changed throughout the hospitalization period, providing for a continuous track of all patients admitted. (a) Label for an identified patient. (b) Label for an unidentified patient. The opening “70” denotes that the number is temporary
The practice of using blood components for transfusions allows giving transfusions of type O packed cells and type AB fresh frozen plasma to the patient without knowing his blood type. Following typing of the patient’s blood, all products should be supplied according to the patient’s blood type. There is no need to stick to O type blood even if 10 units or more were transfused. The reason for this is simple – once packed red blood cells are used, the amount of O type plasma transfused is dismal. Since type AB fresh frozen plasma is transfused when the blood type is unknown, it contains no anti-A or anti-B antibodies, and there is no risk for hemolytic reaction when blood products are further given according to typed blood. Blood supply should be administered according to the massive transfusion protocol when applicable (Table 6.2; Fig. 6.2) and this protocol should be modified according to the specific patient’s need, once lab results are available. In a combined organ trauma that includes brain trauma, a rapid decline of fibrinogen level has been observed and, therefore, early use of cryoprecipitate should be considered before lab results are received. Fibrinogen level should be kept above 150 mg/dl in an actively bleeding patient, and platelet count should be maintained within the range of 100,000/μl in a patient with uncontrolled bleeding. Once blood type results are available, blood products should be administered according to the original blood type.
6.6 Massive-Transfusion Protocols
In recent years, many trauma centers have initiated transfusion protocols. This has been shown to shorten the time for blood products supply and release the trauma team from exact calculations of blood product ratios. It is important to identify early those trauma victims who are massively bleeding. The Joint Theater Trauma System Clinical Practice Guidelines point out several risk factors suggestive of the need for massive transfusion. They include systolic blood pressure <110 mm Hg, heart rate >105 bpm, hematocrit < 32%, pH < 7.25, INR >1.4 and NIR derived O2 saturation <75%. Patients with three of the risk factors have a 70% probability of requiring massive transfusion, and patients with four risk factors have an approximately 85% risk of necessitating massive transfusion. The transfusion service at the Parkland Memorial Hospital in Dallas, Texas, suggested a cooperative effort between the departments of pathology, anesthesiology, and trauma surgery to develop a massive transfusion protocol which was implemented on a trial basis in 2004. The original protocol included five PRBC and two thawed FFP that were dispensed every 30 min from the blood bank. An apheresis platelet unit equal to 6 units of pooled random donor platelets was added to every other shipment and 10 units of cryoprecipitate were included in every third shipment. Recombinant factor VIIa was included in every third shipment [28]. The use of a massive transfusion protocol reduced the turnover time for the first shipment to less than 10 min and the time between the first and second shipment was reduced from 42 to 18 min [28].
Current protocols try to prevent coagulopathy by using a 1:1 ratio or 2:3 ratio of FFP to PRBC. Early preemptive transfusion of cryoprecipitate and platelets is included as part of the protocol and, in some centers, co-transfusion of activated factor VII is added as well. The American Joint Theater Trauma System Clinical Practice Guidelines available on the internet include an example of a massive transfusion protocol that includes a 1:1:1:1 ratio of PRBC, FFP, platelet unit, and cryoprecipitate, and also suggests consideration of Factor VII (Table 6.3). There are two schools of thought regarding the use of massive transfusion protocols (MTP). One implies an automatic discharge of blood products every fixed time from initiation of the protocol. The downside of this strategy might be an accumulation of blood products at the trauma bay and operation theater. The other school supports supply by demand, meaning that following activation of the protocol the blood bank technicians should have the FFP and cryoprecipitate thawed and ready for shipment. Thus, the products are immediately supplied on demand of the resuscitating team. Personally, I prefer the second school which allows better control of the blood product supply and refrains from the accumulation of blood supply at the trauma bay where several injured are simultaneously treated and blood products could be mixed up and misgiven.
6.7 Use of Activated Recombinant Factor VIIa (rFVIIa) and Antifibrinolytic Drugs
The limitation of replacement therapy for profusely bleeding trauma patients suggests the need for additional approaches to the treatment of coagulopathic bleeding. Recombinant factor VIIa is approved worldwide for the treatment of bleeding in patients with hemophilia A or B with inhibitors to coagulation factors VIII or IX. It is thought to work locally at the site of tissue injury where it binds to an exposed tissue factor. This binding activates factor X to Xa and generates a small amount of thrombin. Thrombin activates platelet factors V and VIII to further binding factor VIIa, more prothrombin to thrombin, and generates fibrin from fibrinogen. The interest in the use of recombinant factor VIIa for trauma injuries was initiated by a case report by Kenet et al. who treated a 19-year old soldier with an abdominal penetrating gunshot wound that inflicted tears in the IVC and massive paravertebral muscle damage [17]. This patient with uncontrollable bleeding and an injury severity score of 25 stopped bleeding abruptly and recovered. A later report by Martinowitz et al. of 35 cases showed shortening of prothrombin time and partial thromboplastin time. Cessation of bleeding was achieved in 26 of 36 patients (72%) with a survival rate of 61% [23]. Several randomized control trials evaluating the use of rFVIIa for reduction of bleeding in patients with brain trauma and general trauma revealed conflicting results. In a randomized placebo-controlled study, Boffard et al. [1] evaluated the amount of blood required within 48 h of administration of factor VIIa. The eligibility criterion was the administration of 6 units of PRBC within 4 h of admission. In a group of 158 patients with blunt injury, a reduction of required blood products by 2.6 units compared with controls was demonstrated following factor VIIa administration (p = 0.02). However, in a cohort of 143 patients with penetrating wounds, administration of VIIa did not result in significant reduction of PRBC units transfused [1]. A study of the effect of factor VIIa on the outcome of patients with intracranial hemorrhage also yielded conflicting results. In a cohort of 400 patients, a double blind placebo-controlled trial using a single dose of 40, 80, 160 μg/Kg of factor VIIa or placebo revealed a limited growth of hematomas which was dose-dependent, as well as reduced mortality and improved functional outcome, despite a small increase in thromboembolic adverse events [24]. Unfortunately, a larger phase III study revealed no improvement in bleeding, mortality, or severe disability at the end of the study period (day 90) [26]. Rizoli et al. performed a subgroup analysis of trauma patients treated in a randomized controlled study of three doses of factor VIIa (n = 60) versus placebo (n = 76). The treated group had significantly less episodes of ARDS, a comparable death rate, and fewer patients with multi-organ failure [32].
Regarding the use of recombinant Factor VIIa in combat injury, the level of evidence ranges from case reports to case series; however, no prospective randomized trials have been performed. Spinella et al. [38] retrospectively reviewed a database from a combat support hospital in Baghdad from December 2003 to October 2005, including 124 patients who had massive transfusions of ≥10 PRBC within 24 h. Forty-nine of these patients received rFVIIa and 75 did not. Roughly, 2/3 of the patients had explosion-inflicted injuries and one third had gunshot injuries. The group that did not receive Factor VIIa had a lower mean systolic blood pressure on admission (92 vs 105 mmHg) and received a substantially lower quantity of cryoprecipitate and fresh whole blood. The patients who did not receive FVIIa had a higher 24-h and 30-day mortality rate (35% vs 14%; p = 0.01) (51% vs 31%; p = 0.03), respectively, compared to those who received this product. It is of interest that the mortality rate caused by bleeding did not significantly differ in these groups [38]. Perkins et al. [29] examined the effect of early (prior to 8 units of PRBC) versus late administration of rFVIIa in a combat support hospital in Iraq. Of 5,334 trauma injuries over 22 months (from January 2004), 6.8% required massive transfusions. One hundred and seventeen of these patients (32%) received rFVIIa. Complete records of the blood transfusions were available for 61 patients, 17 of whom received rFVIIa early and 44 who received VIIa later. Early administration of rFVIIa saved 5 units of blood; however, the mortality rate of one third was identical in both groups of patients [29].
The Canadian National Advisory Committee on Blood and Blood Products has concluded that, until large adequately powered clinical trials are available, demonstrating the benefit of rFVIIa in reducing rates of clinically important endpoints with acceptable risk, the routine use of rFVIIa for such indications cannot be recommended [26]. However, there will still be circumstances when the addition of rFVIIa may be necessary for the management of individual patients. It should be remembered that thromboembolic complications were reported following rFVIIa administration in 9.4% of patients [39]. All cardiovascular events and most cases of mesenteric ischemia were attributed to a combination of rFVIIa and a definable high energy vascular injury [29].
Prior to administration of rFVII, several conditions need to be met in order to obtain an optimal response. Hemostatic and other measures need to be taken to correct coagulopathy, including administration of thawed FFP at a dose of 15 cc/Kg; if fibrinogen is less than 1 Gm/L, then 10 units of cryoprecipitate should be given and patient’s blood pH should be >7.1, since rFVII is not active in lower pH. Anti-fibrinolytic agents have shown to have a beneficial effect in thoracic surgery.
Aprotinin is a direct inhibitor of fibrinolytic enzyme plasmin as well as an inhibitor of kallikrain, trypsin, and factor XII. Tranexmic acid and aminocaproic acid are both inhibitors of plasmin binding to fibrin by occupying the lysine binding site of plasminogen proenzyme [22]. For young patients with no known atherosclerotic disease, the use of anti-fibrinolytic drugs should be considered, especially when increased fibrinolysis is observed.
6.8 Thromboelastography
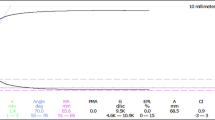
Thromboelastography is a useful point-of-care tool for coagulation evaluation. It is widely employed in thoracic surgery for the identification of coagulopathies. The test is based on continuous documentation of blood clot firmness during the entire coagulation process. Thus, the beginning of clot formation, clot formation kinetics, and maximum clot firmness are assessed as well as its stability or lysis [31, 40]. Thromboelastography provides a dynamic and global assessment of the coagulation process with multiple endpoints reflecting the interaction between platelets, the clotting cascade, and fibrinolysis (Fig. 6.3). Clot initiation time (R time) is correlated with coagulation factors. Kinetics of thrombin generation, clot amplification and propagation are illustrated by an angle k – time to clot formation. Maximal amplitude of the clot is correlated with platelet number, and the time to clot resolution is correlated with fibrinolysis (Fig. 6.4). This bedside apparatus can add significant information regarding primary fibrinolysis, found by Johansson to be the cause of bleeding in 9% of civilian trauma patients [15]. Thromboelastography may be useful when patients are suffering from hypothermia and there is no correlation between the tests performed at 37°C and the actual activity of coagulation factors in the hypothermic patients. In the animal model, hypothermia combined with hemorrhagic shock and fluid resuscitation were found to result in prolonged initial clot formation time (R time) and time to maximum clot formation (K time), as well as impaired maximal amplitudes. Regular thromboelastography performed on TEG does not detect the use of ADP antagonists or arachidonic acid antagonists unless a special kit is used for platelets mapping.
Examples of thromboelastogram patterns and interpretations. This is a representative TEG tracing. The numerical values of the parameters are along the bottom. Each parameter is indicated with a letter, as well as with the units of measurement. Also associated with each parameter are the actual value and the normal range relative to a given sample type
6.9 Conclusions
Transfusion of blood products is an essential component of the treatment of major trauma victims. Verification of the patient’s identity and providing safe blood components both to identified and unidentified injured in mass casualty events require established standard operating procedures. Early identification of patients with trauma-induced coagulopathy may be life-saving, since these patients tend to have higher mortality rates and, therefore, early supply of blood components is helpful for correction of coagulopathy and early damage control resuscitation. As rule of thumb, a higher injury severity score, acidosis, hypothermia, and low blood pressure on admission are predictive factors for trauma-induced coagulopathy. These patients are also candidates for massive transfusion. For patients defined as in need of massive transfusion due to low blood pressure, high pulse rate, or high injury severity score, implementation of massive transfusion protocol is beneficial. Frequent monitoring of CBC, prothrombin time, thromboplastin time, fibrinogen level, and the use of thromboelastogram may be helpful to adjust therapy with PRBC, FFP, cryoprecipitate, rFactor VIIa, and antifibrinolytic agents. It should be emphasized that, while exposure to fresh frozen plasma is life-saving during a massive transfusion or coagulopathy scenario, it could be hazardous and leads to higher rates of multi-organ failure or transfusion-related acute lung injury. Therefore, for patients who do not suffer from major bleeding or coagulopathy, the conservative use of this product should be recommended. For massively bleeding patients, PRBC <14 days old should be used when available. In cases when supply of blood or blood components is short, fresh whole blood may be considered, remembering that this product has not been checked with state-of-the-art tests for transmissible diseases, is not FDA approved, and may be justified only for immediate life-saving scenarios.
“There never was a good war or a bad peace” Benjamin Franklin (1706–1790)
References
Boffard, K.D., Riou, B., Warren, B., et al.: Recombinant factor VIIa as adjunctive therapy for bleeding control in severely injured trauma patients: two parallel randomized, placebo-controlled, double-blind clinical trials. J. Trauma 59, 8–18 (2005)
Borgman, M.A., Spinella, P.C., Perkins, J.G., et al.: The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J. Trauma 63, 805–813 (2007)
Brohi, K., Singh, J., Heron, M., Coats, T.: Acute traumatic coagulopathy. J. Trauma 54, 1127–1130 (2003)
Como, J.J., Dutton, R.P., Scalea, T.M., et al.: Blood transfusion rates in the care of acute trauma. Transfusion 44, 809–813 (2004)
Cosgriff, N., Moore, E.E., Sauaia, A., et al.: Predicting life-threatening coagulopathy in the massively transfused trauma patient: hypothermia and acidoses revisited. J. Trauma 42, 857–862 (1997)
Crosby, W.H., Howard, J.M.: The hematologic response to wounding and to resuscitation accomplished by large transfusions of stored blood; a study of battle casualties in Korea. Blood 9, 439–460 (1954)
Dann, E.J., Bonstein, L., Arbov, L., et al.: Blood bank protocols for large-scale civilian casualty events: experience from terrorist bombing in Israel. Transfus. Med. 17, 135–139 (2007)
Dann, E.J., Michaelson, M., Barzelay, M., et al.: Transfusion medicine during the summer of 2006: lessons learned in northern Israel. Transfus. Med. Rev. 22, 70–76 (2008)
Gonzalez, E.A., Moore, F.A., Holcomb, J.B., et al.: Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J. Trauma 62, 112–119 (2007)
Hess, J.R., Brohi, K., Dutton, R.P., et al.: The coagulopathy of trauma: a review of mechanisms. J. Trauma 65, 748–754 (2008)
Hess, J.R., Holcomb, J.B., Hoyt, D.B.: Damage control resuscitation: the need for specific blood products to treat the coagulopathy of trauma. Transfusion 46, 685–686 (2006)
Hirshberg, A., Dugas, M., Banez, E.I., et al.: Minimizing dilutional coagulopathy in exsanguinating hemorrhage: a computer simulation. J. Trauma 54, 454–463 (2003)
Ho, A.M., Dion, P.W., Cheng, C.A., et al.: A mathematical model for fresh frozen plasma transfusion strategies during major trauma resuscitation with ongoing hemorrhage. Can. J. Surg. 48, 470–478 (2005)
Huber-Wagner, S., Qvick, M., Mussack, T., et al.: Massive blood transfusion and outcome in 1062 polytrauma patients: a prospective study based on the Trauma Registry of the German Trauma Society. Vox Sang. 92, 69–78 (2007)
Johansson, P.I.: The blood bank: from provider to partner in treatment of massively bleeding patients. Transfusion 47, 176S–183S (2007)
Kauvar, D.S., Holcomb, J.B., Norris, G.C., Hess, J.R.: Fresh whole blood transfusion: a controversial military practice. J. Trauma 61, 181–184 (2006)
Kenet, G., Walden, R., Eldad, A., Martinowitz, U.: Treatment of traumatic bleeding with recombinant factor VIIa. Lancet 354, 1879 (1999)
Kiel, F.: Development of a blood program in Vietnam. Mil. Med. 131, 1469–1482 (1966)
Lelkens, C.C., Koning, J.G., de Kort, B., et al.: Experiences with frozen blood products in the Netherlands military. Transfus. Apher. Sci. 34, 289–298 (2006)
Maegele, M., Lefering, R., Yucel, N., et al.: Early coagulopathy in multiple injury: an analysis from the German Trauma Registry on 8724 patients. Injury 38, 298–304 (2007)
Malone, D.L., Hess, J.R., Fingerhut, A.: Massive transfusion practices around the globe and a suggestion for a common massive transfusion protocol. J. Trauma 60, S91–S96 (2006)
Mannucci, P.M., Levi, M.: Prevention and treatment of major blood loss. N Engl J. Med. 356, 2301–2311 (2007)
Martinowitz, U., Michaelson, M.: Guidelines for the use of recombinant activated factor VII (rFVIIa) in uncontrolled bleeding: a report by the Israeli Multidisciplinary rFVIIa Task Force. J. Thromb. Haemost. 3, 640–648 (2005)
Mayer, S.A., Brun, N.C., Begtrup, K., et al.: Recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J. Med. 352, 777–785 (2005)
Mendelson, J.A.: The use of whole blood and blood volume expanders in U.S.medical facilities in Vietnam 1966–1971. J. Trauma 15, 1–13 (1975)
Moltzan, C.J., Anderson, D.A., Callum, J., et al.: The evidence for the use of recombinant factor VIIa in massive bleeding: development of a transfusion policy framework. Transfus. Med. 18, 112–120 (2008)
Niles, S.E., McLaughlin, D.F., Perkins, J.G., et al.: Increased mortality associated with the early coagulopathy of trauma in combat casualties. J. Trauma 64, 1459–1465 (2008)
O’Keeffe, T., Refaai, M., Tchorz, K., et al.: A massive transfusion protocol to decrease blood component use and costs. Arch. Surg. 143, 686–691 (2008)
Perkins, J.G., Schreiber, M.A., Wade, C.E., Holcomb, J.B.: Early versus late recombinant factor VIIa in combat trauma patients requiring massive transfusion. J. Trauma 62, 1095–1101 (2007)
Pfeffermann, R., Rozin, R.R., Durst, A.L., Marin, G.: Modern war surgery: operations in an evacuation hospital during the October 1973 Arab-Israeli war. J. Trauma 16, 694–703 (1976)
Rai, R., Tuddenham, E., Backos, M., et al.: Thromboelastography, whole-blood haemostasis and recurrent miscarriage. Hum. Reprod. 18, 2540–2543 (2003)
Rizoli, S.B., Boffard, K.D., Riou, B., et al.: Recombinant activated factor VII as an adjunctive therapy for bleeding control in severe trauma patients with coagulopathy: subgroup analysis from two randomized trials. Crit. Care 10, R178 (2006)
Robertson, L.B. Further observation on the results of blood transfusion in war surgery, with special reference to the results in primary haemorrhage. Br. Med. J. 2, 679–682 (1917)
Shinar, E., Yahalom, V., Silverman, B.G.: Meeting blood requirements following terrorist attacks: the Israeli experience. Curr. Opin. Hematol. 13, 452–456 (2006)
Sperry, J.L., Ochoa, J.B., Gunn, S.R., et al.: An FFP:PRBC transfusion ratio >/=1:1.5 is associated with a lower risk of mortality after massive transfusion. J. Trauma 65, 986–993 (2008)
Spinella, P.C., Perkins, J.G., Grathwohl, K.W., et al.: Warm fresh whole blood is independently associated with improved survival for patients with combat-related traumatic injuries. J. Trauma 66, S69–S76 (2009)
Spinella, P.C., Perkins, J.G., Grathwohl, K.W., et al.: Risks associated with fresh whole blood and red blood cell transfusions in a combat support hospital. Crit. Care Med. 35, 2576–2581 (2007)
Spinella, P.C., Perkins, J.G., McLaughlin, D.F., et al.: The effect of recombinant activated factor VII on mortality in combat-related casualties with severe trauma and massive transfusion. J. Trauma 64, 286–294 (2008)
Thomas, G.O., Dutton, R.P., Hemlock, B., et al.: Thromboembolic complications associated with factor VIIa administration. J. Trauma 62, 564–569 (2007)
Vanek, T., Jares, M., Snircova, J., Maly, M.: Fibrinolysis in coronary artery surgery: detection by thromboelastography. Interact. Cardiovasc. Thorac. Surg. 6, 700–704 (2007)
Watson, G.A., Sperry, J.L., Rosengart, M.R., et al.: Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. J. Trauma 67, 221–230 (2009)
Zhou, J.Y., Bi, X.X., Tang, R.C., Huang, C.Y.: Evaluation of in vivo viability of human platelets cryopreserved at -80 degrees C by using SCID mouse model. Zhongguo Shi Yan Xue Ye Xue Za Zhi 17, 802–804 (2009)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Berlin Heidelberg
About this chapter
Cite this chapter
Dann, E.J. (2011). Hemotransfusion in Combat Trauma. In: Lerner, A., Soudry, M. (eds) Armed Conflict Injuries to the Extremities. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-16155-1_6
Download citation
DOI: https://doi.org/10.1007/978-3-642-16155-1_6
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-16154-4
Online ISBN: 978-3-642-16155-1
eBook Packages: MedicineMedicine (R0)