Abstract
Exercise can improve cognitive function and the outcome of neurodegenerative diseases like Alzheimer’s disease. This effect has been linked to the increased expression of brain-derived neurotrophic factor (BDNF). However, the underlying molecular mechanisms driving the elevation of this neurotrophin remain unknown. Recently, we have reported a PGC-1α-FNDC5/irisin pathway that is activated by exercise in the hippocampus in mice and induces a neuroprotective gene program, including Bdnf. This review will focus on FNDC5 and its secreted form “irisin,” a newly discovered myokine, its role in the nervous system and its therapeutic potential. In addition, we will briefly discuss the role of other exercise-induced myokines in positive brain effects.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Introduction
Exercise, especially endurance exercise, is known to have beneficial effects on brain health and cognitive function (Cotman et al. 2007; Mattson 2012; Voss et al. 2013). This improvement in cognitive function with exercise has been most prominently observed in the aging population (Colcombe and Kramer 2003). Exercise has also been reported to ameliorate outcomes in neurological diseases such as depression, epilepsy, stroke, and Alzheimer’s and Parkinson’s disease (Ahlskog 2011; Arida et al. 2008; Buchman et al. 2012; Russo-Neustadt et al. 1999; Zhang et al. 2012). The effects of exercise on the brain are most apparent in the hippocampus and the dentate gyrus, a part of the brain involved in learning and memory. Specific beneficial effects of exercise in the brain have been reported to include increases in the size of, and blood flow to, the hippocampus in humans and morphological changes in dendrites and dendritic spines, increased synapse plasticity and, importantly, de novo neurogenesis in the dentate gyrus in various mouse models of exercise (Cotman et al. 2007; Mattson 2012). De novo neurogenesis in the adult brain is observed in only two areas; the dentate gyrus of the hippocampus is one of them. Exercise is one of the few known stimuli of this de novo neurogenesis (Kobilo et al. 2011).
One important molecular mediator of these beneficial responses in the brain to exercise is the induction of neurotrophins/growth factors, most notably brain-derived neurotrophic factor (BDNF). In animal models, BDNF is induced in various regions of the brain with exercise, most robustly in the hippocampus (Cotman et al. 2007). BDNF promotes many aspects of brain development, including neuronal cell survival, differentiation, migration, dendritic arborization, synaptogenesis and plasticity (Greenberg et al. 2009; Park and Poo 2013). In addition, BDNF is essential for synaptic plasticity, hippocampal function, and learning (Kuipers and Bramham 2006). Highlighting the relevance of BDNF in humans, individuals carrying the Val66Met mutation in the Bdnf gene exhibit decreased secretion of BDNF, decreased volume of specific brain regions, deficits in episodic memory function and increased anxiety and depression (Egan et al. 2003; Hariri et al. 2003). Blocking BDNF signaling with anti-TrkB antibodies attenuates the exercise-induced improvement of acquisition and retention of a spatial learning task, as well as the exercise-induced expression of synaptic proteins (Vaynman et al. 2004, 2006). However, the underlying mechanism that induces BDNF in exercise remains incompletely understood.
We recently described a role for the newly discovered “exercise-hormone,” FNDC5 (Bostrom et al. 2012), and its secreted form, “irisin,” in the protective effects of exercise on the brain. Fndc5 expression is induced by exercise in the hippocampus in mice; it can, in turn, activate BDNF and other neuroprotective genes (Wrann et al. 2013). Importantly, peripheral delivery of FNDC5 to the liver via adenoviral vectors, resulting in elevated blood irisin, induces expression of Bdnf and other neuroprotective genes in the hippocampus. These data indicate that either irisin itself can cross the blood-brain-barrier to induce gene expression changes or irisin induces a “factor x” that can, which has significant implications for irisin as a novel therapeutic target. This review will examine previous literature about FNDC5/irisin as well as its therapeutic potential for treating neurodegenerative disease.
Discovery of FNDC5/Irisin
In 2002, two groups independently cloned a novel gene termed either PeP or, alternatively, Frcp2, that contained a fibronectin type III (FNIII) domain; it is now named FNDC5 (Ferrer-Martinez et al. 2002; Teufel et al. 2002). Recently, our group identified FNDC5 as a PGC-1α-dependent myokine that is secreted from muscle during exercise and induces some major metabolic benefits of exercise (Bostrom et al. 2012).
FNDC5 is a glycosylated type I membrane protein. It contains a N-terminal signal peptide [amino acid (aa) 1-28], a FNIII domain (aa 33–124), a transmembrane domain (aa 150–170), and a cytoplasmic tail (aa 171–209) (www.uniporot.org) (Fig. 1). The secreted form of FNDC5 contains 112 amino acids (aa 29-140), named irisin, and is generated by proteolytic cleavage and is released into the circulation. The protease/sheddase responsible for that cleavage has not yet been identified. Irisin has been crystallized and its structure has been solved (Schumacher et al. 2013). Interestingly, the FNIII-like domain shows an unusual confirmation, with a continuous intersubunit beta-sheet dimer, that has not been previously described for any other FNIII protein. Subsequent biochemical experiments confirmed the existence of irisin (bacterial recombinant) as a homodimer.
Analysis of Irisin Peptides by Mass Spectrometry. (a) Scheme of the murine FNDC5 protein structure (top) and murine irisin protein structure (bottom). SP signal peptide, H hydrophobic domain, C cytoplasmic domain. (b) Murine FNDC5 amino acid sequence with corresponding domains colored. The irisin sequence is underlined
Irisin in Humans
Irisin is a highly conserved polypeptide across mammals and is, in fact, 100% identical in mice and humans (Bostrom et al. 2012). Such a high degree of conservation is often the result of evolutionary pressure to conserve function. Interestingly, the human FNDC5 has an atypical start of translation, ATA in place of ATG, as compared to mouse Fndc5. While it is now known that a few percent of eukaryotic mRNAs begin translation with non-ATG start codons (Ingolia et al. 2011; Ivanov et al. 2011; Peabody 1989) and are often associated with regulation on the translational level (Chang and Wang 2004; Starck et al. 2012), recent reports (Albrecht et al. 2015; Raschke et al. 2013) have argued that this ATA codon in human FNDC5 was a “null mutation” or a “myth” and, as a result, human irisin would not be produced. Furthermore, many reports from other groups measuring irisin in humans by Western blot or ELISA have suggested results to be artefacts of poor antibody specificity (Albrecht et al. 2015; Erickson 2013; Raschke et al. 2013), even though an earlier study had detected irisin circulating in human plasma using mass spectrometry, an unbiased method independent of the quality of existing antibodies (Jedrychowski et al. 2015; Lee et al. 2014). To identify and quantify irisin in human plasma, we used targeted mass spectrometry with control peptides enriched with stable isotopes as internal standards. This precise, state-of-the-art method demonstrated that human irisin was mainly translated from its non-canonical ATA start codon (Jedrychowski et al. 2015). In addition, it showed that, in sedentary individuals, irisin circulated at ~3.6 ng/ml and that it was significantly increased in individuals undergoing aerobic interval training. This study determined that, at the atomic level, human irisin exists, and is regulated by certain forms of aerobic exercise.
FNDC5/Irisin in Exercise
FNDC5/irisin was first described as an exercise-induced myokine by Bostrom et al. in 2012, where they observed upregulation of Fndc5 gene expression in skeletal muscle and increased serum irisin levels after prolonged endurance exercise in both mice and humans. Increasing the circulating levels of irisin by overexpression of FNDC5 from adenoviral vectors in the liver led to an increase in “browning” of the white inguinal adipose tissue, i.e., the upregulation of mitochondrial gene expression, especially of Ucp1, and an increased glucose tolerance in mice. Both are among the major metabolic benefits of endurance exercise. By now, there are around 50 published papers that investigate the role of FNDC5 and/or irisin in exercise, including both rodent studies and clinical trials in humans. Induction of Fndc5 mRNA in skeletal muscle by endurance exercise has been confirmed in several studies in both mice (Quinn et al. 2015; Tiano et al. 2015; Wrann et al. 2013) and humans (Albrecht et al. 2015; Lecker et al. 2012; Norheim et al. 2014) using qPCR or RNA sequencing. As with all clinical studies, there are a lot of variables to consider, such as retrospective studies vs. interventional trials, age and fitness level of the subjects and, most importantly, the type of exercise protocol used and time point of sampling. However, a consensus is building, supported by studies that reported positive associations between irisin plasma level and exercise, performed early sampling and high intensity training protocols levels (Daskalopoulou et al. 2014; Huh et al. 2014; Kraemer et al. 2014; Malcolm Eaton et al. 2017; Norheim et al. 2014). Plasma levels of irisin and BDNF have been shown to be positively correlated with cognitive function in endurance athletes (Belviranli et al. 2016).
The brief rise in circulating irisin levels after exercise is suggestive of an acute shedding event of irisin during exercise. There is little to no evidence thus far that FNDC5 or irisin is upregulated by resistance exercise in mouse or human This is not unexpected, since endurance exercise activates PGC-1α1, which has been shown to be the upstream regulator of Fndc5 gene expression, whereas resistance exercise activates a different isoform of PGC-1α1, PGC-1α4 (Ruas et al. 2012).
FNDC5/Irisin in Neuronal Development
Fndc5 is highly expressed in the brain, including the Purkinje cells of the cerebellum (Dun et al. 2013; Ferrer-Martinez et al. 2002; Teufel et al. 2002). Irisin, the shed form of FNDC5, was identified in human cerebrospinal fluid by Western blot (Piya et al. 2014). In addition, immunoreactivity against the extracellular domain of FNDC5/irisin was detected in human hypothalamic sections, especially paraventricular neurons (Piya et al. 2014). Other tissues with high FNDC5 levels include skeletal muscle and the heart. Fndc5 gene expression increases during differentiation of rat pheochromocytoma-derived PC12 cells into neuron-like cells (Ostadsharif et al. 2011). FNDC5 levels are enhanced after the differentiation of human embryonic stem cell-derived neural cells into neurons (Ghahrizjani et al. 2015) as well as during the maturation of primary cortical neurons in culture and during brain development in vivo (Wrann et al. 2013).
Knockdown of FNDC5 in neuronal precursors impaired their development into mature neurons (and astrocytes), suggesting a developmental role for FNDC5 in neurons (Hashemi et al. 2013). Despite this finding, forced expression of FNDC5 during neuronal precursor formation from mouse embryonic stem cells increased mature neuronal markers (Map2, b-tubulinIII and Neurocan) and an astrocyte marker (GFAP) and BDNF. However, overexpression of FNDC5 in undifferentiated mouse embryonic stem cells did not have these effects, indicating that FNDC5 supports neural differentiation rather than lineage commitment (Forouzanfar et al. 2015). Pharmacological doses of recombinant irisin increased cell proliferation in the mouse H19-7 hippocampal cell line (Moon et al. 2013). Furthermore, forced expression of FNDC5 in primary cortical neurons increased cell survival in culture, whereas knockdown of FNDC5 had the opposite effect (Wrann et al. 2013).
FNDC5/Irisin: Other Effects in the CNS
The group of Dr. Mulholland (University of Michigan) had taken in interest in the central nervous effects of irisin. In a first study, they injected irisin either into third ventricle of rats or intravenously measured the effects on blood pressure and cardiac contractibility (Zhang et al. 2015a). Central administration of irisin activated neurons in the paraventricular nuclei of the hypothalamus, as indicated by increased c-fos immunoreactivity. Central irisin administration also increased both blood pressure and cardiac contractibility. In contrast, i.v. injection of irisin reduced blood pressure in both control and spontaneously hypertensive rats. In a second study, Zhang et al. showed that central treatment of rats with irisin-Fc led to an increase in physical activity as measured as total travel distance, ambulatory counts and time, and vertical counts and time compared to control animals receiving IgG Fc peptide (Zhang et al. 2015b). In addition, the centrally applied irisin also induced significant increases in oxygen consumption, carbon dioxide production and heat production, indicating an increase in metabolic activity- possibly through SNS activation. Similarly, intra-hypothalamic injection of irisin decreased food intake in rats possibly by stimulating anorexigenic peptides and inhibiting dopamine, norepinephrine and orexin-A (Ferrante et al. 2016).
A recent study investigated the neuroprotective potential of irisin treatments in cerebral ischemia. Irisin improved survival of cultured PC12 neuronal cells in oxygen glucose deprivation. I.v. injection of recombinant irisin reduced brain infarct and edema volume and improved the neurological score in MCAO stroke model (Li et al. 2017). Systemic irisin administration has also been shown to ameliorate depressive-like behavior in a chronic unpredictable stress model in rats (Wang and Pan 2016).
Exercise Induces Hippocampal BDNF Through a PGC-1α/FNDC5 Pathway
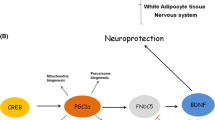
In a recent study, we have shown that FNDC5 is also elevated in the hippocampus of mice undergoing endurance exercise regimen of 30-days free-wheel running. Neuronal Fndc5 gene expression is regulated by PGC-1α and Pgc1a−/− mice show reduced Fndc5 expression in the brain. Forced expression of FNDC5 in primary cortical neurons increases Bdnf expression, whereas RNAi-mediated knockdown of FNDC5 reduces Bdnf. Importantly, peripheral delivery of FNDC5 to the liver via adenoviral vectors, resulting in elevated blood irisin, induces expression of Bdnf and other neuroprotective genes in the hippocampus. Taken together, our findings link endurance exercise and the important metabolic mediators, PGC-1α and FNDC5, with BDNF expression in the brain. Interestingly, a recent study investigating the effects of the flavonoid quercetin hypobaric hypoxia, reported that quercetin administration to hyperbaric hypoxic rats increased expression of PGC-1α, FNDC5, and BDNF in the hippocampus (Liu et al. 2015). While more research will be required to determine whether the FNDC5/irisin protein can improve cognitive function in animals, this study suggests that a hormone administered peripherally could induce some of the effects of endurance exercise on the brain (Fig. 2).
Other Circulating Factors from the Muscle
While FNDC5/irisin is a very interesting molecule that holds therapeutic promise, this is not to say that we think that FNDC5/irisin captures all the benefits of exercise on the brain or that is the only important secreted molecule from muscle in exercise. In fact, other such molecules have been described, including BDNF, IGF-1, and VEGF, kynurenic acid, cathepsin B, as well as a variety of cytokines and chemokines, to name a few (Agudelo et al. 2014; Moon et al. 2016; Phillips et al. 2014; Voss et al. 2013). We expect that in the future, additional molecules will be discovered and some may reach their full therapeutic potential.
References
Agudelo LZ, Femenia T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V, Correia JC, Izadi M, Bhat M, Schuppe-Koistinen I, Pettersson AT, Ferreira DM, Krook A, Barres R, Zierath JR, Erhardt S, Lindskog M, Ruas JL (2014) Skeletal muscle PGC-1alpha1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 159:33–45
Ahlskog JE (2011) Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology 77:288–294
Albrecht E, Norheim F, Thiede B, Holen T, Ohashi T, Schering L, Lee S, Brenmoehl J, Thomas S, Drevon CA, Erickson HP, Maak S (2015) Irisin – a myth rather than an exercise-inducible myokine. Sci Rep 5:8889
Arida RM, Cavalheiro EA, da Silva AC, Scorza FA (2008) Physical activity and epilepsy: proven and predicted benefits. Sports Med 38:607–615
Belviranli M, Okudan N, Kabak B, Erdogan M, Karanfilci M (2016) The relationship between brain-derived neurotrophic factor, irisin and cognitive skills of endurance athletes. Phys Sportsmed 44:290–296
Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM (2012) A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481:463–468
Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA (2012) Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology 78:1323–1329
Chang KJ, Wang CC (2004) Translation initiation from a naturally occurring non-AUG codon in Saccharomyces cerevisiae. J Biol Chem 279:13778–13785
Colcombe S, Kramer AF (2003) Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci 14:125–130
Cotman CW, Berchtold NC, Christie LA (2007) Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci 30:464–472
Daskalopoulou SS, Cooke AB, Gomez YH, Mutter AF, Filippaios A, Mesfum ET, Mantzoros CS (2014) Plasma irisin levels progressively increase in response to increasing exercise workloads in young, healthy, active subjects. Eur J Endocrinol 171:343–352
Dun SL, Lyu RM, Chen YH, Chang JK, Luo JJ, Dun NJ (2013) Irisin-immunoreactivity in neural and non-neural cells of the rodent. Neuroscience 240:155–162
Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR (2003) The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112:257–269
Erickson HP (2013) Irisin and FNDC5 in retrospect: an exercise hormone or a transmembrane receptor? Adipocyte 2:289–293
Ferrante C, Orlando G, Recinella L, Leone S, Chiavaroli A, Di Nisio C, Shohreh R, Manippa F, Ricciuti A, Vacca M, Brunetti L (2016) Central inhibitory effects on feeding induced by the adipo-myokine irisin. Eur J Pharmacol 791:389–394
Ferrer-Martinez A, Ruiz-Lozano P, Chien KR (2002) Mouse PeP: a novel peroxisomal protein linked to myoblast differentiation and development. Dev Dyn 224:154–167
Forouzanfar M, Rabiee F, Ghaedi K, Beheshti S, Tanhaei S, Shoaraye Nejati A, Jodeiri Farshbaf M, Baharvand H, Nasr-Esfahani MH (2015) Fndc5 overexpression facilitated neural differentiation of mouse embryonic stem cells. Cell Biol Int 39:629–637
Ghahrizjani FA, Ghaedi K, Salamian A, Tanhaei S, Nejati AS, Salehi H, Nabiuni M, Baharvand H, Nasr-Esfahani MH (2015) Enhanced expression of FNDC5 in human embryonic stem cell-derived neural cells along with relevant embryonic neural tissues. Gene 557:123–129
Greenberg ME, Xu B, Lu B, Hempstead BL (2009) New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci 29:12764–12767
Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR (2003) Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci 23:6690–6694
Hashemi MS, Ghaedi K, Salamian A, Karbalaie K, Emadi-Baygi M, Tanhaei S, Nasr-Esfahani MH, Baharvand H (2013) Fndc5 knockdown significantly decreased neural differentiation rate of mouse embryonic stem cells. Neuroscience 231:296–304
Huh JY, Mougios V, Kabasakalis A, Fatouros I, Siopi A, Douroudos II, Filippaios A, Panagiotou G, Park KH, Mantzoros CS (2014) Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. J Clin Endocrinol Metab 99:E2154–E2161
Ingolia NT, Lareau LF, Weissman JS (2011) Ribosome profiling of mouse embryonic stem cells reveals the complexity and dynamics of mammalian proteomes. Cell 147:789–802
Ivanov IP, Firth AE, Michel AM, Atkins JF, Baranov PV (2011) Identification of evolutionarily conserved non-AUG-initiated N-terminal extensions in human coding sequences. Nucleic Acids Res 39:4220–4234
Jedrychowski MP, Wrann CD, Paulo JA, Gerber KK, Szpyt J, Robinson MM, Nair KS, Gygi SP, Spiegelman BM (2015) Detection and quantitation of circulating human irisin by tandem mass spectrometry. Cell Metab 22:734–740
Kobilo T, Liu QR, Gandhi K, Mughal M, Shaham Y, van Praag H (2011) Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem 18:605–609
Kraemer RR, Shockett P, Webb ND, Shah U, Castracane VD (2014) A transient elevated irisin blood concentration in response to prolonged, moderate aerobic exercise in young men and women. Horm Metab Res 46:150–154
Kuipers SD, Bramham CR (2006) Brain-derived neurotrophic factor mechanisms and function in adult synaptic plasticity: new insights and implications for therapy. Curr Opin Drug Discov Dev 9:580–586
Lecker SH, Zavin A, Cao P, Arena R, Allsup K, Daniels KM, Joseph J, Schulze PC, Forman DE (2012) Expression of the irisin precursor FNDC5 in skeletal muscle correlates with aerobic exercise performance in patients with heart failure. Circ Heart Fail 5:812–818
Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, Perron RM, Werner CD, Phan GQ, Kammula US et al (2014) Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab 19:302–309
Li DJ, Li YH, Yuan HB, Qu LF, Wang P (2017) The novel exercise-induced hormone irisin protects against neuronal injury via activation of the Akt and ERK1/2 signaling pathways and contributes to the neuroprotection of physical exercise in cerebral ischemia. Metabolism 68:31–42
Liu P, Zou D, Yi L, Chen M, Gao Y, Zhou R, Zhang Q, Zhou Y, Zhu J, Chen K et al (2015) Quercetin ameliorates hypobaric hypoxia-induced memory impairment through mitochondrial and neuron function adaptation via the PGC-1alpha pathway. Restor Neurol Neurosci 33:143–157
Malcolm Eaton CG, Barry J, Safdar A, Bishop D, Jonathan P (2017) Little impact of a single bout of high-intensity interval exercise and short-term interval training on interleukin-6, FNDC5, and METRNL mRNA expression in human skeletal muscle. J Sport Health Sci 4–0
Mattson MP (2012) Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab 16:706–722
Moon HS, Dincer F, Mantzoros CS (2013) Pharmacological concentrations of irisin increase cell proliferation without influencing markers of neurite outgrowth and synaptogenesis in mouse H19-7 hippocampal cell lines. Metabolism 62:1131–1136
Moon HY, Becke A, Berron D, Becker B, Sah N, Benoni G, Janke E, Lubejko ST, Greig NH, Mattison JA, Duzel E, van Praag H (2016) Running-induced systemic cathepsin B secretion is associated with memory function. Cell Metab 24:332–340
Norheim F, Langleite TM, Hjorth M, Holen T, Kielland A, Stadheim HK, Gulseth HL, Birkeland KI, Jensen J, Drevon CA (2014) The effects of acute and chronic exercise on PGC-1alpha, irisin and browning of subcutaneous adipose tissue in humans. FEBS J 281:739–749
Ostadsharif M, Ghaedi K, Hossein Nasr-Esfahani M, Mojbafan M, Tanhaie S, Karbalaie K, Baharvand H (2011) The expression of peroxisomal protein transcripts increased by retinoic acid during neural differentiation. Differentiation 81:127–132
Park H, Poo MM (2013) Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci 14:7–23
Peabody DS (1989) Translation initiation at non-AUG triplets in mammalian cells. J Biol Chem 264:5031–5035
Phillips C, Baktir MA, Srivatsan M, Salehi A (2014) Neuroprotective effects of physical activity on the brain: a closer look at trophic factor signaling. Front Cell Neurosci 8:170
Piya MK, Harte AL, Sivakumar K, Tripathi G, Voyias PD, James S, Sabico S, Al-Daghri NM, Saravanan P, Barber TM, Kumar S, Vatish M, McTernan PG (2014) The identification of irisin in human cerebrospinal fluid: influence of adiposity, metabolic markers, and gestational diabetes. Am J Physiol Endocrinol Metab 306:E512–E518
Quinn LS, Anderson BG, Conner JD, Wolden-Hanson T (2015) Circulating irisin levels and muscle FNDC5 mRNA expression are independent of IL-15 levels in mice. Endocrine 50:368–377
Raschke S, Elsen M, Gassenhuber H, Sommerfeld M, Schwahn U, Brockmann B, Jung R, Wisloff U, Tjonna AE, Raastad T, Hallén J, Norheim F, Drevon CA, Romacho T, Eckardt K, Eckel J (2013) Evidence against a beneficial effect of irisin in humans. PLoS One 8:e73680
Ruas JL, White JP, Rao RR, Kleiner S, Brannan KT, Harrison BC, Greene NP, Wu J, Estall JL, Irving BA, Lanza IR, Rasbach KA, Okutsu M, Nair KS, Yan Z, Leinwand LA, Spiegelman BM (2012) A PGC-1alpha isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 151:1319–1331
Russo-Neustadt A, Beard RC, Cotman CW (1999) Exercise, antidepressant medications, and enhanced brain derived neurotrophic factor expression. Neuropsychopharmacology 21:679–682
Schumacher MA, Chinnam N, Ohashi T, Shah RS, Erickson HP (2013) The structure of irisin reveals a novel intersubunit beta-sheet fibronectin type III (FNIII) dimer: implications for receptor activation. J Biol Chem 288:33738–33744
Starck SR, Jiang V, Pavon-Eternod M, Prasad S, McCarthy B, Pan T, Shastri N (2012) Leucine-tRNA initiates at CUG start codons for protein synthesis and presentation by MHC class I. Science 336:1719–1723
Teufel A, Malik N, Mukhopadhyay M, Westphal H (2002) Frcp1 and Frcp2, two novel fibronectin type III repeat containing genes. Gene 297:79–83
Tiano JP, Springer DA, Rane SG (2015) SMAD3 negatively regulates serum irisin and skeletal muscle FNDC5 and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1alpha) during exercise. J Biol Chem 290:7671–7684
Vaynman S, Ying Z, Gomez-Pinilla F (2004) Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci 20:2580–2590
Vaynman SS, Ying Z, Yin D, Gomez-Pinilla F (2006) Exercise differentially regulates synaptic proteins associated to the function of BDNF. Brain Res 1070:124–130
Voss MW, Vivar C, Kramer AF, van Praag H (2013) Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci 17:525–544
Wang S, Pan J (2016) Irisin ameliorates depressive-like behaviors in rats by regulating energy metabolism. Biochem Biophys Res Commun 474:22–28
Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM (2013) Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell Metab 18:649–659
Zhang Q, Wu Y, Zhang P, Sha H, Jia J, Hu Y, Zhu J (2012) Exercise induces mitochondrial biogenesis after brain ischemia in rats. Neuroscience 205:10–17
Zhang W, Chang L, Zhang C, Zhang R, Li Z, Chai B, Li J, Chen E, Mulholland M (2015a) Central and peripheral irisin differentially regulate blood pressure. Cardiovasc Drugs Ther 29:121–127
Zhang W, Chang L, Zhang C, Zhang R, Li Z, Chai B, Li J, Chen E, Mulholland M (2015b) Irisin: a myokine with locomotor activity. Neurosci Lett 595:7–11
Acknowledgements
This work was supported by the NIH (NS087096). I also thank Dr. Mark Jedrychowski for support with the art work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made. The images or other third party material in this book are included in the book's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the book's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2017 The Author(s)
About this chapter
Cite this chapter
Islam, M.R., Young, M.F., Wrann, C.D. (2017). The Role of FNDC5/Irisin in the Nervous System and as a Mediator for Beneficial Effects of Exercise on the Brain. In: Spiegelman, B. (eds) Hormones, Metabolism and the Benefits of Exercise. Research and Perspectives in Endocrine Interactions. Springer, Cham. https://doi.org/10.1007/978-3-319-72790-5_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-72790-5_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-72789-9
Online ISBN: 978-3-319-72790-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)