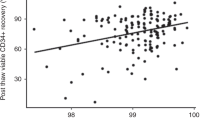
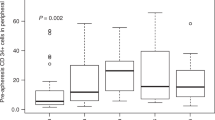
Abstract
Hematopoietic cell transplants (HCTs) have been performed for decades, and the number of both autologous and allogeneic transplants is constantly increasing. Mobilized peripheral blood (PB) hematopoietic progenitor cells (HPCs) are the most common cell source for adult patients, replacing almost completely the traditional bone marrow (BM) graft. Cryopreservation of PB HPC graft allows collecting and storing the product for later use. Cryopreservation is an essential practice for autologous products but can also be helpful for allogeneic one. The cryopreservation process itself can decrease cell viability and potency, and some of the reagents that are used to protect cells during cryopreservation can have toxic effects at thaw. Maintaining functional and viable HPCs is fundamental for HCT success. Thawing approaches of cryopreserved PB HPC should either minimize cell exposure time to toxic elements or include measures to rapidly remove them after thawing.
Similar content being viewed by others
References
AABB AAoTB, American Red Cross, American Society for Blood and Marrow, Transplantation ASfA, America’s Blood Centers, College of American Pathologists, Cord Blood Association, Foundation for the Accreditation of, Cellular Therapy I, International NetCord Foundation, International Society for Cellular Therapy, JACIE Accreditation Office, Program NMD (2016) Circular of information for the use of cellular therapy products. Bethesda, MD, AABB
Akkök CA, Holte MR, Tangen JM, Ostenstad B, Bruserud O (2009) Hematopoietic engraftment of dimethyl sulfoxide-depleted autologous peripheral blood progenitor cells. Transfusion 49(2):354–361
Alessandrino P, Bernasconi P, Caldera D et al (1999) Adverse events occurring during bone marrow or peripheral blood progenitor cell infusion: analysis of 126 cases. Bone Marrow Transplant 23(6):533–537
Anchordoguy TJ, Carpenter JF, Crowe JH, Crowe LM (1992) Temperature-dependent perturbation of phospholipid bilayers by dimethylsulfoxide. Biochim Biophys Acta 1104(1):117–122
Arakawa T, Carpenter JF, Kita YA, Crowe JH (1990) The basis for toxicity of certain cryoprotectants: a hypothesis. Cryobiology 27:401–415
Bakken AM, Bruserud O, Abrahamsen JF (2003) No differences in colony formation of peripheral blood stem cells frozen with 5% or 10% dimethyl sulfoxide. J Hematother Stem Cell Res 12(3):351–358
Baust JG, Gao D, Baust JM (2009) Cryopreservation: an emerging paradigm change. Organogenesis 5(3):90–96
Benekli M, Anderson B, Wentling D, Bernstein S, Czuczman M, McCarthy P (2000) Severe respiratory depression after dimethylsulphoxide-containing autologous stem cell infusion in a patient with AL amyloidosis. Bone Marrow Transplant 25(12):1299–1301
Bothner U, Georgieff M, Vogt NH (1998) Assessment of the safety and tolerance of 6% hydroxyethyl starch (200/0.5) solution: a randomized, controlled epidemiology study. Anesth Analg 86(4):850–855
Bowman CA, Yu M, Cottler-Fox M (1996) Evaluation of methods for preparing and thawing cryopreserved CD34+ and CD34− cell lines for use as reagents in flow cytometry of hematopoietic progenitor cells. Transfusion 36(11-12):985–988
Brave M, Farrell A, Ching Lin S et al (2010) FDA review summary: Mozobil in combination with granulocyte colony-stimulating factor to mobilize hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation. Oncology 78(3-4):282–288
Broxmeyer HE, Srour EF, Hangoc G, Cooper S, Anderson SA, Bodine DM (2003) High-efficiency recovery of functional hematopoietic progenitor and stem cells from human cord blood cryopreserved for 15 years. Proc Natl Acad Sci U S A 100(2):645–650
Calmels B, Houzé P, Hengesse JC, Ducrot T, Malenfant C, Chabannon C (2003) Preclinical evaluation of an automated closed fluid management device: Cytomate, for washing out DMSO from hematopoietic stem cell grafts after thawing. Bone Marrow Transplant 31(9):823–828
Cordoba R, Arrieta R, Kerguelen A, Hernandez-Navarro F (2007) The occurrence of adverse events during the infusion of autologous peripheral blood stem cells is related to the number of granulocytes in the leukapheresis product. Bone Marrow Transplant 40(11):1063–1067
Davis JM, Rowley SD, Braine HG, Piantadosi S, Santos GW (1990) Clinical toxicity of cryopreserved bone marrow graft infusion. Blood 75(3):781–786
Dhodapkar M, Goldberg SL, Tefferi A, Gertz MA (1994) Reversible encephalopathy after cryopreserved peripheral blood stem cell infusion. Am J Hematol 45(2):187–188
Donnenberg AD, Koch EK, Griffin DL et al (2002) Viability of cryopreserved BM progenitor cells stored for more than a decade. Cytotherapy 4(2):157–163
Fahy GM (1986) The relevance of cryoprotectant “toxicity” to cryobiology. Cryobiology 23(1):1–13
Foïs E, Desmartin M, Benhamida S et al (2007) Recovery, viability and clinical toxicity of thawed and washed haematopoietic progenitor cells: analysis of 952 autologous peripheral blood stem cell transplantations. Bone Marrow Transplant 40(9):831–835
Hoyt R, Szer J, Grigg A (2000) Neurological events associated with the infusion of cryopreserved bone marrow and/or peripheral blood progenitor cells. Bone Marrow Transplant 25(12):1285–1287
Kao GS (2009) Assessment of collection quality. In: Areman EM, Loper K (eds) Cellular therapy: principles, methods, and regulations. AABB, Bethesda, MD, pp 291–302
Keating GM (2011) Plerixafor: a review of its use in stem-cell mobilization in patients with lymphoma or multiple myeloma. Drugs 71(12):1623–1647
Kessinger A, Schmit-Pokorny K, Smith D, Armitage J (1990) Cryopreservation and infusion of autologous peripheral blood stem cells. Bone Marrow Transplant 5(Suppl 1):25–27
Khera N, Jinneman J, Storer BE et al (2012) Limiting the daily total nucleated cell dose of cryopreserved peripheral blood stem cell products for autologous transplantation improves infusion-related safety with no adverse impact on hematopoietic engraftment. Biol Blood Marrow Transplant 18(2):220–228
Lemarie C, Calmels B, Malenfant C et al (2005) Clinical experience with the delivery of thawed and washed autologous blood cells, with an automated closed fluid management device: CytoMate. Transfusion 45(5):737–742
López-Jiménez J, Cerveró C, Muñoz A et al (1994) Cardiovascular toxicities related to the infusion of cryopreserved grafts: results of a controlled study. Bone Marrow Transplant 13(6):789–793
Lovelock JE, Bishop MW (1959) Prevention of freezing damage to living cells by dimethyl sulphoxide. Nature 183(4672):1394–1395
Mazur P (2004) Principles of cryobiology. In: Fuller BJ, Lane N, Benson E (eds) Life in the frozen state. CRC, Boca Raton, FL, pp 3–66
Mazur P, Leibo SP, Chu EH (1972) A two-factor hypothesis of freezing injury. Evidence from Chinese hamster tissue-culture cells. Exp Cell Res 71(2):345–355
Pagano MB, Harmon C, Cooling L et al (2016) Use of hydroxyethyl starch in leukocytapheresis procedures does not increase renal toxicity. Transfusion 56(11):2848–2856
Pasquini M, Zhu X (2015) Current uses and outcomes of hematopoietic stem cell transplantation: CIBMTR summary slides. http://www.cibmtr.org
Perotti CG, Del Fante C, Viarengo G et al (2004) A new automated cell washer device for thawed cord blood units. Transfusion 44(6):900–906
Rapoport AP, Rowe JM, Packman CH, Ginsberg SJ (1991) Cardiac arrest after autologous marrow infusion. Bone Marrow Transplant 7(5):401–403
Rodrigues JP, Paraguassú-Braga FH, Carvalho L, Abdelhay E, Bouzas LF, Porto LC (2008) Evaluation of trehalose and sucrose as cryoprotectants for hematopoietic stem cells of umbilical cord blood. Cryobiology 56(2):144–151
Rodríguez L, Velasco B, García J, Martín-Henao GA (2005) Evaluation of an automated cell processing device to reduce the dimethyl sulfoxide from hematopoietic grafts after thawing. Transfusion 45(8):1391–1397
Rowley S, MacLeod B, Heimfeld S, Holmberg L, Bensinger W (1999) Severe central nervous system toxicity associated with the infusion of cryopreserved PBSC components. Cytotherapy 1(4):311–317
Rubinstein P, Dobrila L, Rosenfield RE et al (1995) Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci U S A 92(22):10119–10122
Runckel DN, Swanson JR (1980) Effect of dimethyl sulfoxide on serum osmolality. Clin Chem 26(12):1745–1747
Samoszuk M, Reid ME, Toy PT (1983) Intravenous dimethylsulfoxide therapy causes severe hemolysis mimicking a hemolytic transfusion reaction. Transfusion 23(5):405
Sánchez-Salinas A, Cabañas-Perianes V, Blanquer M et al (2012) An automatic wash method for dimethyl sulfoxide removal in autologous hematopoietic stem cell transplantation decreases the adverse effects related to infusion. Transfusion 52(11):2382–2386
Sauer-Heilborn A, Kadidlo D, McCullough J (2004) Patient care during infusion of hematopoietic progenitor cells. Transfusion 44(6):907–916
Scerpa MC, Daniele N, Landi F et al (2011) Automated washing of human progenitor cells: evaluation of apoptosis and cell necrosis. Transfus Med 21(6):402–407
Smith DM, Weisenburger DD, Bierman P, Kessinger A, Vaughan WP, Armitage JO (1987) Acute renal failure associated with autologous bone marrow transplantation. Bone Marrow Transplant 2(2):195–201
Snyder EL, Haley NR (eds) (2004) Cellular therapy: a physician’s handbook, 1st edn. Bethesda, MD, AABB
Spurr EE, Wiggins NE, Marsden KA, Lowenthal RM, Ragg SJ (2002) Cryopreserved human haematopoietic stem cells retain engraftment potential after extended (5-14 years) cryostorage. Cryobiology 44(3):210–217
Svalgaard JD, Haastrup EK, Reckzeh K et al (2016) Low-molecular-weight carbohydrate Pentaisomaltose may replace dimethyl sulfoxide as a safer cryoprotectant for cryopreservation of peripheral blood stem cells. Transfusion 56(5):1088–1095
Syme R, Bewick M, Stewart D, Porter K, Chadderton T, Glück S (2004) The role of depletion of dimethyl sulfoxide before autografting: on hematologic recovery, side effects, and toxicity. Biol Blood Marrow Transplant 10(2):135–141
Vercueil A, Grocott MP, Mythen MG (2005) Physiology, pharmacology, and rationale for colloid administration for the maintenance of effective hemodynamic stability in critically ill patients. Transfus Med Rev 19(2):93–109
Wagner JE, Barker JN, DeFor TE et al (2002) Transplantation of unrelated donor umbilical cord blood in 102 patients with malignant and nonmalignant diseases: influence of CD34 cell dose and HLA disparity on treatment-related mortality and survival. Blood 100(5):1611–1618
Windrum P, Morris TC, Drake MB, Niederwieser D, Ruutu T, Subcommittee ECLWPC (2005) Variation in dimethyl sulfoxide use in stem cell transplantation: a survey of EBMT centres. Bone Marrow Transplant 36(7):601–603
Woods EJ, Liu J, Derrow CW, Smith FO, Williams DA, Critser JK (2000) Osmometric and permeability characteristics of human placental/umbilical cord blood CD34+ cells and their application to cryopreservation. J Hematother Stem Cell Res 9(2):161–173
Yellowlees P, Greenfield C, McIntyre N (1980) Dimethylsulphoxide-induced toxicity. Lancet 2(8202):1004–1006
Zambelli A, Poggi G, Da Prada G et al (1998) Clinical toxicity of cryopreserved circulating progenitor cells infusion. Anticancer Res 18(6B):4705–4708
Zeisberger SM, Schulz JC, Mairhofer M et al (2011) Biological and physicochemical characterization of a serum- and xeno-free chemically defined cryopreservation procedure for adult human progenitor cells. Cell Transplant 20(8):1241–1257
Zenhäusern R, Tobler A, Leoncini L, Hess OM, Ferrari P (2000) Fatal cardiac arrhythmia after infusion of dimethyl sulfoxide-cryopreserved hematopoietic stem cells in a patient with severe primary cardiac amyloidosis and end-stage renal failure. Ann Hematol 79(9):523–526
Zhang XB, Li K, Yau KH et al (2003) Trehalose ameliorates the cryopreservation of cord blood in a preclinical system and increases the recovery of CFUs, long-term culture-initiating cells, and nonobese diabetic-SCID repopulating cells. Transfusion 43(2):265–272
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Reich-Slotky, R. (2018). Peripheral Blood Hematopoietic Progenitor Cell Graft Thawing. In: Schwartz, J., Shaz, B. (eds) Best Practices in Processing and Storage for Hematopoietic Cell Transplantation . Advances and Controversies in Hematopoietic Transplantation and Cell Therapy. Springer, Cham. https://doi.org/10.1007/978-3-319-58949-7_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-58949-7_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-58948-0
Online ISBN: 978-3-319-58949-7
eBook Packages: MedicineMedicine (R0)