Abstract
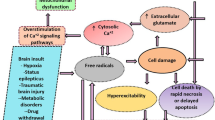
Generalized convulsive status epilepticus is a life-threatening condition with high mortality; it also causes cellular damage. Systemic physiologic changes in blood pressure, oxygenation, and temperature can cause widespread neuronal injury, but even when systemic physiologic parameters are controlled, status epilepticus can cause focal cell loss in the hippocampus. There is progressive loss of GABAergic inhibitory neurotransmission and sustained glutamatergic excitatory neurotransmission during the course of status epilepticus. Glutamate-mediated excitotoxicity is the most likely cause of focal neuronal injury. If status epilepticus continues for more than an hour, these changes can eventually result in hippocampal cell death, probably because N-methyl-d-aspartate (NMDA) glutamate receptors are concentrated in this region. The pattern of cell loss is similar to hypoxic cell damage but is also distinct and identical to the pattern of cell loss found in medial temporal lobe epilepsy due to hippocampal sclerosis. Early treatment of status epilepticus is necessary to prevent irreversible neuronal injury and cell death.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Dodrill CB, Wilensky AJ. Intellectual impairment as an outcome of status epilepticus. Neurology. 1990;40(Suppl 2):23–7.
Aicardi J, Chevrie JJ. Convulsive status epilepticus in infants and children. A study of 239 cases. Epilepsia. 1970;11(2):187–97.
Lothman EW, Bertram EH. Epileptogenic effects of status epilepticus. Epilepsia. 1993;34(Suppl 1):S59–70.
Kapur J. Status epilepticus in epileptogenesis. Curr Opin Neurol. 1999;12(2):191–5.
Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Incidence of status epilepticus in Rochester, Minnesota, 1965–1984. Neurology. 1998;50(3):735–41.
Eriksson KJ, Koivikko MJ. Status epilepticus in children: aetiology, treatment, and outcome. Dev Med Child Neurol. 1997;39(10):652–8.
Gaspard N, Foreman BP, Alvarez V, Cabrera Kang C, Probasco JC, Jongeling AC, et al. Critical Care EEG Monitoring Research Consortium (CCEMRC). New-onset refractory status epilepticus: Etiology, clinical features, and outcome. Neurology. 2015;85(18):1604–13.
Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Risk of unprovoked seizure after acute symptomatic seizure: effect of status epilepticus. Ann Neurol. 1998;44(6):908–12.
Kramer RE, Luders H, Lesser RP, Weinstein MR, Dinner DS, Morris HH, Wyllie E. Transient focal abnormalities of neuroimaging studies during focal status epilepticus. Epilepsia. 1987;28(5):528–32.
Nohria V, Lee N, Tien RD, Heinz ER, Smith JS, DeLong G, et al. Magnetic resonance imaging evidence of hippocampal sclerosis in progression: a case report. Epilepsia. 1994;35(6):1332–6.
Tien RD, Felsberg GJ. The hippocampus in status epilepticus: demonstration of signal intensity and morphologic changes with sequential fast spin-echo MR imaging. Radiology. 1995;194(1):249–56.
Wieshmann UC, Woermann FG, Lemieux L, Free SL, Bartlett PA, Smith SJ, et al. Development of hippocampal atrophy: a serial magnetic resonance imaging study in a patient who developed epilepsy after generalized status epilepticus. Epilepsia. 1997;38(11):1238–41.
Riela AR, Sires BP, Penry JK. Transient magnetic resonance imaging abnormalities during partial status epilepticus. J Child Neurol. 1991;6(2):143–5.
Salmenperä T, Kälviäinen R, Partanen K, Mervaala E, Pitkänen A. MRI volumetry of the hippocampus, amygdala, entorhinal cortex, and perirhinal cortex after status epilepticus. Epilepsy Res. 2000;40(2–3):155–70.
Henry TR, Drury I, Brunberg JA, Pennell PB, McKeever PE, Beydoun A. Focal cerebral magnetic resonance changes associated with partial status epilepticus. Epilepsia. 1994;35(1):35–41.
DeGiorgio CM, Gott PS, Rabinowicz AL, Heck CN, Smith T, Correale J. Neuron-specific enolase, a marker of acute neuronal injury, is increased in complex partial status epilepticus. Epilepsia. 1996;37(7):606–9.
DeGiorgio CM, Heck CN, Rabinowicz AL, Gott PS, Smith T, Correale J. Serum neuron-specific enolase in the major subtypes of status epilepticus. Neurology. 1999;52(4):746–9.
Correale J, Rabinowicz AL, Heck CN, Smith TD, Loskota WJ, DeGiorgio CM. Status epilepticus increases CSF levels of neuron-specific enolase and alters the blood-brain barrier. Neurology. 1998;50(5):1388–91.
Rabinowicz AL, Correale J, Bracht KA, Smith TD, DeGiorgio CM. Neuron-specific enolase is increased after nonconvulsive status epilepticus. Epilepsia. 1995;36(5):475–9.
Meyer Z, Beck E, Shepherd M. Unusually severe lesions in the brain following status epilepticus. J Neurol Neurosurg Psychiatry. 1955;18(1):24–33.
Norman RM. The neuropathology of status epilepticus. Med Sci Law. 1964;4:46–51.
Corsellis JAN, Bruton CJ. Neuropathology of status epilepticus in humans. In: Delgado-Escueta AV, Wasterlain CG, Treiman DM, Porter RJ, editors. Status epilepticus. Advances in Neurology, vol. 34. New York: Raven Press; 1983. p. 129–140.
Nixon J, Bateman D, Moss T. An MRI and neuropathological study of a case of fatal status epilepticus. Seizure. 2001;10(8):588–91.
Albala BJ, Moshé SL, Okada R. Kainic-acid-induced seizures: a developmental study. Brain Res. 1984;315(1):139–48.
Stafstrom CE, Thompson JL, Holmes GL. Kainic acid in the developing brain: status epilepticus and spontaneous recurrent seizures. Brain Res Dev Brain Res. 1992;65(2):227–36.
Cilio MR, Sogawa Y, Cha BH, Liu X, Huang LT, Holmes GL. Long-term effects of status epilepticus in the immature brain are specific for age and model. Epilepsia. 2003;44(4):518–28.
Fujikawa DG, Itabashi HH, Wu A, Shinmei SS. Status epilepticus-induced neuronal loss in humans without systemic complications or epilepsy. Epilepsia. 2000;41(8):981–91.
DeGiorgio CM, Tomiyasu U, Gott PS, Treiman DM. Hippocampal pyramidal cell loss in human status epilepticus. Epilepsia. 1992;33(1):23–7.
Knopman D, Margolis G, Reeves AG. Prolonged focal epilepsy and hypoxemia as a cause of focal brain damage: a case study. Ann Neurol. 1977;1(2):195–8.
Soffer D, Melamed E, Assaf Y, Cotev S. Hemispheric brain damage in unilateral status epilepticus. Ann Neurol. 1986;20(6):737–40.
Kaplan PW. Nonconvulsive status epilepticus: to lump or to split? (abstract). Epilepsia. 1994;35(Suppl 8):9.
Fountain NB, Lothman EW. Pathophysiology of status epilepticus. J Clin Neurophysiol. 1995;12(4):326–42 Review.
Shneker B, Fountain NB. Epilepsy as chronic sequelae following nonconvulsive status epilepticus (abstract). Epilepsia. 2001;42(Suppl 7):147.
Scholtes FB, Reiner WO, Meinardi H. Non-convulsive status epilepticus: causes, treatment and outcome in 65 patients. J Neurol Neurosurg Psychiatry. 1996;61(1):93–5.
Shneker BF, Fountain NB. Assessment of acute morbidity and mortality in nonconvulsive status epilepticus. Neurology. 2003;61(8):1066–73.
Jope RS, Morrisett RA, Snead OC. Characterization of lithium potentiation of pilocarpine-induced status epilepticus in rats. Exp Neurol. 1986;91(3):471–80.
Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L. Limbic seizures produced by pilocarpine in rats: behavioural, electroencephalographic and neuropathological study. Behav Brain Res. 1983;9(3):315–35.
Lothman EW, Collins RC. Kainic acid induced limbic seizures: metabolic, behavioral, electroencephalographic and neuropathological correlates. Brain Res. 1981;218(1–2):299–318.
Lothman EW, Collins RC, Ferrendelli JA. Kainic acid-induced limbic seizures: electrophysiologic studies. Neurology. 1981;31(7):806–12.
Walton NY, Treiman DM. Experimental secondarily generalized convulsive status epilepticus induced by D, L-homocysteine thiolactone. Epilepsy Res. 1988;2(2):79–86.
Todorovic MS, Cowan ML, Balint CA, Sun C, Kapur J. Characterization of status epilepticus induced by two organophosphates in rats. Epilepsy Res. 2012;101(3):268–76.
Deshpande LS, Carter DS, Blair RE, DeLorenzo RJ. Development of a prolonged calcium plateau in hippocampal neurons in rats surviving status epilepticus induced by the organophosphate diisopropylfluorophosphate. Toxicol Sci. 2010;116(2):623–31.
McDonough JH Jr, Dochterman LW, Smith CD, Shih TM. Protection against nerve agent-induced neuropathology, but not cardiac pathology, is associated with the anticonvulsant action of drug treatment. Neurotoxicology. 1995;16(1):123–32.
Ben Ari Y, Lagowska J, Tremblay E, Gal La Salle G. A new model of focal status epilepticus: intra-amygdaloid application of kainic acid elicits repetitive secondarily generalized convulsive seizures. Brain Res. 1979;163(1):176–179.
Furtado MA, Castro OW, Del Vecchio F, de Oliveira JAC, Garcia-Cairasco N. Study of spontaneous recurrent seizures and morphological alterations after status epilepticus induced by intrahippocampal injection of pilocarpine. Epilepsy Behav. 2011;20(2):257–66.
Tanaka T, Kaijima M, Daita G, Ohgami S, Yonemasu Y, Riche D. Electroclinical features of kainic acid-induced status epilepticus in freely moving cats. Microinjection into the dorsal hippocampus. Electroencephalogr Clin Neurophysiol. 1982;54(3):288–300.
Lothman EW, Bertram EH, Bekenstein JW, Perlin JB. Self-sustaining limbic status epilepticus induced by ‘continuous’ hippocampal stimulation: electrographic and behavioral characteristics. Epilepsy Res. 1989;3(2):107–19.
McIntyre DC, Nathanson D, Edson N. A new model of partial status epilepticus based on kindling. Brain Res. 1982;250(1):53–63.
Mazarati AM, Wasterlain CG, Sankar R, Shin D. Self-sustaining status epilepticus after brief electrical stimulation of the perforant path. Brain Res. 1998;801(1–2):251–3.
Kapur J, MacDonald RL. Rapid seizure-induced reduction of benzodiazepine and Zn2+ sensitivity of hippocampal dentate granule cell GABAA receptors. J Neurosci. 1997;17(19):7532–40.
Goodkin HP, Joshi S, Mtchedlishvili Z, Brar J, Kapur J. Subunit-specific trafficking of GABA(A) receptors during status epilepticus. J Neurosci. 2008;28(10):2527–38.
Naylor DE, Liu H, Wasterlain CG. Trafficking of GABAA receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci. 2005;25(34):7724–33.
Kapur J, Lothman EW, DeLorenzo RJ. Loss of GABAA receptors during partial status epilepticus. Neurology. 1994;44(12):2407–8.
Kapur J, Coulter DA. Experimental status epilepticus alters gamma-aminobutyric acid type A receptor function in CA1 pyramidal neurons. Ann Neurol. 1995;38(6):893–900.
Terunuma M, Xu J, Vithlani M, Sieghart W, Kittler J, Pangalos M, et al. Deficits in phosphorylation of GABA(A) receptors by intimately associated protein kinase C activity underlie compromised synaptic inhibition during status epilepticus. J Neurosci. 2008;28(2):376–84.
Joshi S, Rajasekaran K, Hawk KM, Brar J, Ross BM, Tran CA, et al. Phosphatase inhibition prevents the activity-dependent trafficking of GABAA receptors during status epilepticus in the young animal. Epilepsia. 2015;56(9):1355–65.
Walton NY, Treiman DM. Response of status epilepticus induced by lithium and pilocarpine to treatment with diazepam. Exp Neurol. 1988;101(2):267–75.
Jones DM, Esmaeil N, Maren S, MacDonald RL. Characterization of pharmacoresistance to benzodiazepines in the rat Li-pilocarpine model of status epilepticus. Epilepsy Res. 2002;50(3):301–12.
Goodkin HP, Yeh JL, Kapur J. Status epilepticus increases the intracellular accumulation of GABAA receptors. J Neurosci. 2005;25(23):5511–20.
Naylor DE, Liu H, Niquet J, Wasterlain CG. Rapid surface accumulation of NMDA receptors increases glutamatergic excitation during status epilepticus. Neurobio Dis. 2013;54:225–38.
Borris DJ, Bertram EH, Kapur J. Ketamine controls prolonged status epilepticus. Epilepsy Res. 2000;42(2–3):117–22.
Yen W, Williamson J, Bertram EH, Kapur J. A comparison of three NMDA receptor antagonists in the treatment of prolonged status epilepticus. Epilepsy Res. 2004;59(1):43–50.
Martin BS, Kapur J. A combination of ketamine and diazepam synergistically controls refractory status epilepticus induced by cholinergic stimulation. Epilepsia. 2008;49(2):248–55.
Rice AC, DeLorenzo RJ. N-Methyl-aspartate receptor activation regulates refractoriness of status epilepticus to diazepam. Neuroscience. 1999;93(1):117–23.
Fujikawa DG. The temporal evolution of neuronal damage from pilocarpine-induced status epilepticus. Brain Res. 1996;725(1):11–22.
Honchar MP, Olney JW, Sherman WR. Systemic cholinergic agents induce seizures and brain damage in lithium-treated rats. Science. 1983;220(4594):323–5.
Clifford DB, Olney JW, Maniotis A, Collins RC, Zorumski CF. The functional anatomy and pathology of lithium-pilocarpine and high-dose pilocarpine seizures. Neuroscience. 1987;23(3):953–68.
Dingledine R, Varvel NH, Dudek FE. When and how do seizures kill neurons, and is cell death relevant to epileptogenesis? Adv Exp Med Biol. 2014;813:109–22.
Turski WA, Cavalheiro EA, Bortolotto ZA, Mello LM, Schwarz M, Turski L. Seizures produced by pilocarpine in mice: a behavioral, electroencephalographic and morphological analysis. Brain Res. 1984;321(2):237–53.
Schwob JE, Fuller T, Price JL, Olney JW. Widespread patterns of neuronal damage following systemic or intracerebral injections of kainic acid: a histological study. Neuroscience. 1980;5(6):991–1014.
Buckmaster PS, Dudek FE. Neuron loss, granule cell axon reorganization, and functional changes in the dentate gyrus of epileptic kainate-treated rats. J Comp Neurol. 1997;385(3):385–404.
Fujikawa DG, Daniels AH, Kim JS. The competitive NMDA receptor antagonist CGP 40116 protects against status epilepticus-induced neuronal damage. Epilepsy Res. 1994;17(3):207–19.
Bertram EH, Lothman EW, Lenn NJ. The hippocampus in experimental chronic epilepsy: a morphometric analysis. Ann Neurol. 1990;27(1):43–8.
Du F, Eid T, Lothman EW, Kohler C, Schwarcz R. Preferential neuronal loss in layer III of the medial entorhinal cortex in rat models of temporal lobe epilepsy. J Neurosci. 1995;15(10):6301–13.
Kobayashi M, Buckmaster PS. Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. J Neurosci. 2003;23(6):2440–52.
Sun C, Mtchedlishvili Z, Bertram EH, Erisir A, Kapur J. Selective loss of dentate hilar interneurons contributes to reduced synaptic inhibition of granule cells in an electrical stimulation-based animal model of temporal lobe epilepsy. J Comp Neurol. 2007;500(5):876–93.
Mathern GW, Bertram EH 3rd, Babb TL, Pretorius JK, Kuhlman PA, Spradlin S, Mendoza D. In contrast to kindled seizures, the frequency of spontaneous epilepsy in the limbic status model correlates with greater aberrant fascia dentata excitatory and inhibitory axon sprouting, and increased staining for N-methyl-d-aspartate, AMPA and GABA(A) receptors. Neuroscience. 1997;77(4):1003–19.
Obenaus A, Esclapez M, Houser CR. Loss of glutamate decarboxylase mRNA-containing neurons in the rat dentate gyrus following pilocarpine-induced seizures. J Neurosci. 1993;13(10):4470–85.
Cavalheiro EA, Silva DF, Turski WA, Calderazzo-Filho LS, Bortolotto ZA, Turski L. The susceptibility of rats to pilocarpine-induced seizures is age-dependent. Brain Res. 1987;465(1–2):43–58.
Lynch M, Sayin U, Bownds J, Janumpalli S, Sutula T. Long-term consequences of early postnatal seizures on hippocampal learning and plasticity. Eur J Neurosci. 2000;12(7):2252–64.
Kubová H, Mares P, Suchomelová L, Brozek G, Druga R, Pitkänen A. Status epilepticus in immature rats leads to behavioural and cognitive impairment and epileptogenesis. Eur J Neurosci. 2004;19(12):3255–65.
Sankar R, Shin DH, Liu H, Mazarati A. Pereira de Vasconcelos A, Wasterlain CG. Patterns of status epilepticus-induced neuronal injury during development and long-term consequences. J Neurosci. 1998;18(20):8382–93.
Blennow G, Brierley JB, Meldrum BS, Siesjö BK. Epileptic brain damage: the role of systemic factors that modify cerebral energy metabolism. Brain. 1978;101(4):687–700.
Fountain NB. Status epilepticus: risk factors and complications. Epilepsia. 2000;41(Suppl 2):S23–30.
Teitelbaum JS, Zatorre RJ, Carpenter S, Gendron D, Evans AC, Gjedde A, Cashman NR. Neurologic sequelae of domoic acid intoxication due to the ingestion of contaminated mussels. N Engl J Med. 1990;322(25):1781–7.
Perl TM, Bedard L, Kosatsky T, Hockin JC, Todd EC, Remis RS. An outbreak of toxic encephalopathy caused by eating mussels contaminated with domoic acid. N Engl J Med. 1990;322(25):1775–80.
Sparenborg S, Brennecke LH, Jaax NK, Braitman DJ. Dizocilpine (MK-801) arrests status epilepticus and prevents brain damage induced by soman. Neuropharmacology. 1992;31(4):357–68.
Brandt C, Potschka H, Loscher W, Ebert U. N-methyl-d-aspartate receptor blockade after status epilepticus protects against limbic brain damage but not against epilepsy in the kainate model of temporal lobe epilepsy. Neuroscience. 2003;118(3):727–40.
Chen J, Zheng G, Guo H, Shi ZN. Role of endoplasmic reticulum stress via the PERK signaling pathway in brain injury from status epilepticus. J Mol Neurosci. 2014;53(4):677–83.
Sokka AL, Putkonen N, Mudo G, Pryazhnikov E, Reijonen S, Khiroug L, et al. Endoplasmic reticulum stress inhibition protects against excitotoxic neuronal injury in the rat brain. J Neurosci. 2007;27(4):901–8.
Vezzani A, Dingledine R, Rossetti AO. Immunity and inflammation in status epilepticus and its sequelae: possibilities for therapeutic application. Expert Rev Neurother. 2015;15(9):1081–92.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer Science+Business Media LLC
About this chapter
Cite this chapter
Fountain, N.B., Joshi, S. (2018). Neuropathology of Generalized Convulsive Status Epilepticus. In: Drislane, F., Kaplan MBBS, P. (eds) Status Epilepticus. Current Clinical Neurology. Springer, Cham. https://doi.org/10.1007/978-3-319-58200-9_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-58200-9_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-58198-9
Online ISBN: 978-3-319-58200-9
eBook Packages: MedicineMedicine (R0)