Abstract
The hypothalamic-pituitary system is essential for maintaining life and controlling systemic homeostasis. However, it can be negatively affected by various diseases, resulting in life-long serious symptoms.
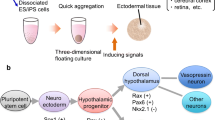
Pluripotent stem cells, such as embryonic stem (ES) cells and induced pluripotent stem (iPS) cells, differentiate into neuroectodermal progenitors when cultured as floating aggregates under serum-free conditions.
Recent results have shown that strict removal of exogenous patterning factors during the early differentiation period induces efficient generation of rostral hypothalamic-like progenitors from mouse ES cell-derived neuroectodermal cells. The use of growth factor-free, chemically defined medium was critical for this induction. The ES cell-derived hypothalamic-like progenitors generated rostral-dorsal hypothalamic neurons, in particular magnocellular vasopressinergic neurons, which release hormones upon stimulation.
We subsequently reported efficient self-formation of three-dimensional adenohypophysis tissues in aggregate cultures of mouse ES cells. The ES cells were stimulated to differentiate into non-neural head ectoderm and hypothalamic neuroectoderm in adjacent layers within the aggregate, followed by treatment with a Sonic Hedgehog agonist. Self-organization of Rathke’s pouch-like structures occurred at the interface of the two epithelia in vivo, and various endocrine cells, including corticotrophs and somatotrophs, were subsequently produced. The corticotrophs efficiently secreted adrenocorticotropic hormone in response to corticotropin-releasing hormone. Furthermore, when engrafted in vivo, these cells rescued systemic glucocorticoid levels in hypopituitary mice.
The present study aimed to prepare hypothalamic and pituitary tissues from human pluripotent stem cells and establish effective transplantation techniques for future clinical applications. Preliminary results indicated differentiation using human ES/iPS cells, and the culture method replicated stepwise embryonic differentiation. Therefore, these methods could potentially be used as developmental and disease models as well as for future regenerative medicine.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Embryonic Stem
- Embryonic Stem Cell
- Human Embryonic Stem Cell
- Mouse Embryonic Stem Cell
- Corticotropin Release Hormone
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The hypothalamus and adenohypophysis maintain physiological homeostasis by controlling the endocrine system. A collection of studies exploring their development and function has shown that they are essential for the regulation of vital functions. However, their regeneration remains largely unclear.
Recently, somatic stem cells have been recognized as a major source for tissue maintenance and regeneration. A 2005 study reported that somatic stem cells exist in the adenohypophysis (Chen et al. 2005). Subsequent studies have discussed their functions during early postnatal pituitary maturation (Fauquier et al. 2001; Kikuchi et al. 2007; Chen et al. 2009; Gremeaux et al. 2012; Mollard et al. 2012), after pituitary damage (Luque et al. 2011; Fu et al. 2012; Langlais et al. 2013), and in pituitary tumorigenesis (Gaston-Massuet et al. 2011; Andoniadou et al. 2012; Garcia-Lavandeira et al. 2012; Li et al. 2012).
In addition to somatic stem cells, studies have focused on embryonic stem (ES) cells and induced pluripotent stem (iPS) cells. These pluripotent stem cells exhibit self-renewal properties and pluripotent differentiation. Therefore, they have attracted attention as a cell source for differentiated tissues in clinical applications.
A Need for Hypothalamus and Adenohypophysis Regenerative Medicine
The hypothalamus and adenohypophysis are located in adjacent regions, and they coordinate functions as the center for the endocrine systems. Following dysfunction, patients experience various systemic symptoms. Current treatment primarily consists of hormone replacement therapy, but various factors can complicate the proper dose. Drug administration cannot precisely mimic the circadian or stress-induced changes of hormone requirements. For example, we have reported that some patients with central diabetes insipidus show unstable serum Na levels, resulting in a poor prognosis (Arima et al. 2014). This instability is caused by the lack of positive and negative control systems, which is characteristic of hormone-producing cells. As for hypopituitarism, it has been reported that adrenal crisis occurs in a substantial proportion of society, and adrenal crisis-associated mortality is not negligible, even in educated patients (Hahner et al. 2015). Furthermore, adrenocorticotropic hormone (ACTH)-dependent adrenal insufficiency, as well as high-dose hydrocortisone treatment, serves as a predictor for acromegaly-associated mortality (Sherlock et al. 2009; Ben-Shlomo 2010). Taken together, these factors indicate that there are many prospects for pituitary regenerative medicine.
Mouse ES Cells
The establishment of mouse pluripotent ES cells significantly contributed to the advancement of biology and medicine. In 1981, Evans and Kaufman successfully established mouse ES cells from the inner cell mass of mouse blastocyst-stage embryos (Evans and Kaufman 1981). Various knock-out and knock-in mice have been established using genetically modified ES cells, which have contributed to our understanding of gene functions (Bernstein and Breitman 1989; Babinet and Cohen-Tannoudji 2001).
There are two reasons for the use of mouse ES cells, rather than human ES cells, in our recent studies. One reason is the short developmental period; the duration of mouse fetal development is about 20 days, which is much shorter than the 300 days of human development. Therefore, mouse ES cells are suitable for establishing novel differentiation methods with numerous trial-and-error processes. Another reason is the similarity between mouse and human cells. For example, the retinal differentiation method from human ES cells (Nakano et al. 2012) was established based on a previous report using mouse ES cells (Eiraku et al. 2011). Although the inducing culture methods differ considerably, their key principles are similar. The fundamental processes of mouse ES cells appear to be applicable to human ES cells.
Pituitary Gland Embryology
The adenohypophysis, which corresponds to the anterior pituitary gland, contains several types of endocrine cells that secrete factors including adrenocorticotropic hormone (ACTH), growth hormone (GH), prolactin (PRL), thyroid-stimulating hormone, luteinizing hormone, and follicle-stimulating hormone. The posterior pituitary gland consists of axons and terminals of hypothalamic neurons, i.e., vasopressin and oxytocin neurons. The development of the adenohypophysis is a complex process. During early development, the adenohypophysis anlage originates as a placode in the non-neural ectoderm adjacent to the anterior neural plate (Fig. 1a). Both the adenohypophysis placode and hypothalamic anlage interact with each other. Accordingly, the thickened placode invaginates and subsequently detaches from the oral ectoderm to form a hollowed vesicle, termed “Rathke’s pouch” (Zhu et al. 2007; Fig. 1b). The molecular nature of this local inductive interaction during this initial phase of pituitary formation remains elusive, but FGF and BMP signals appear to be involved (Takuma et al. 1998; Brinkmeier et al. 2007).
Three-Dimensional ES Cell Culture
Organ formation during embryogenesis consists of complicated processes that involve various local interactions between different tissues or cells. Despite this complexity, organogenesis can be modeled in vivo. Our colleagues established a three-dimensional culture method for ES cells called “serum-free culture of embryoid body-like aggregates with quick re-aggregation (SFEBq)” (Watanabe et al. 2005; Eiraku et al. 2008). The culture method is quite simple. First, the quality of maintenance for undifferentiated ES cells is very important. For SFEBq culture, maintained ES cells are dissociated to single cells in trypsin or something similar. The cells are then quickly aggregated using low-cell adhesion 96-well plates in differentiation medium suitable for each differentiation purpose.
This culture method is suitable for induction of various ectodermal derivatives from ES cells. In SEFBq cultures, the ES cell aggregates exhibit self-organization (Sasai et al. 2012) and spontaneous formation of a highly ordered structure or patterning. This floating culture has revealed intrinsic programs that drive locally autonomous modes of organogenesis and homeostasis. Using the SFEBq method, mesencephalic dopamine neurons (Kawasaki et al. 2002; Morizane et al. 2006), cortex neurons (Eiraku et al. 2008; Danjo et al. 2011; Kadoshima et al. 2013), the optic cup (Eiraku et al. 2011; Ikeda et al. 2005; Osakada et al. 2008), cerebellar neurons (Muguruma et al. 2010), and hippocampal neurons (Sakaguchi et al. 2015) have been generated from mouse and human ES cells.
Induction of Hypothalamic Neurons from Mouse ES Cells
Using SFEBq cultures, hypothalamic neurons such as vasopressin-positive neurons have been induced from mouse ES cells (Wataya et al. 2008). The differentiation occurs efficiently when the ES cell aggregates are cultured in growth factor-free, chemically defined medium (gfCDM). Strict removal of exogenous patterning factors during early differentiation steps induces efficient generation of rostral hypothalamic-like progenitors (Rax(+)/Six3(+)/Vax1(+); these combinations are characteristic for hypothalamic precursors) in mouse ES cell aggregates. The use of gfCDM is critical. For example, even the presence of exogenous insulin, which is commonly used in cell culture, strongly inhibits differentiation via the Akt-dependent pathway. The ES cell-derived hypothalamic progenitors generate Otp(+)/Brn2(+) neuronal precursors (characteristic of rostral-dorsal hypothalamic neurons) and subsequent magnocellular vasopressinergic neurons that release vasopressin upon stimulation. Additionally, differentiation markers of rostral-“ventral” hypothalamic precursors and neurons have been induced from ES cell-derived Rax(+) progenitors by treatment with Sonic Hedgehog (Shh).
Thus, in the absence of exogenous growth factors in the medium, ES cell-derived neuroectodermal cells spontaneously differentiated into rostral (particularly rostral-dorsal) hypothalamic-like progenitors, which generated characteristic hypothalamic neuroendocrine neurons in a stepwise fashion, as observed in vivo. These findings indicated that, instead of the addition of inductive signals, minimization of exogenous patterning signaling played a key role in rostral hypothalamic specification of neural progenitors derived from pluripotent cells. This work also showed that the default fate of mouse ES cells is the rostral hypothalamus (Wataya et al. 2008).
Two-Layer Formation In Vitro Is the First Step of Adenohypohysis Differentiation
We next established an in vitro differentiation method for the anterior pituitary (38). Rathke’s pouch is formed as a result of interactions between the hypothalamus and neighboring oral ectoderm (Zhu et al. 2007). To recapitulate embryonic pituitary development, we co-induced these two tissues within one ES cell aggregate.
Previous results have shown hypothalamic differentiation from mouse ES cells (37). Mouse ES cells can be induced to differentiate into hypothalamic cells when cultured as floating aggregates using the SFEBq method with gfCDM. Therefore, the present study used a technical modification to co-induce oral ectodermal differentiation in addition to hypothalamic differentiation.
We attempted to slightly shift positional information so that the oral ectoderm co-existed with hypothalamic tissues (Suga et al. 2011). As shown in Fig. 1a, the oral ectoderm is generated from the rostral and midline region adjacent to the hypothalamic region in the mouse embryo. Therefore, the rostral and midline shifting information was relevant for mouse ES cell aggregates in the SFEBq culture. We tested many culture conditions known to affect early ectodermal patterning. We ultimately identified two conditions that efficiently induced oral ectoderm. One condition was the addition of bone morphogenetic protein 4 (BMP4). However, treatment with 0.5 μM BMP4 strongly inhibited hypothalamic neuron differentiation instead of inducing oral ectodermal differentiation. The other condition was high-density cell aggregation (10,000 cells per aggregate instead of 3,000 in SFEBq culture), which we refer to as large cell aggregation (LCA; Fig. 2a). In the LCA culture, both the oral ectoderm (Pitx1/2+) and hypothalamic tissues co-existed within one aggregate (Fig. 2b).
LCA culture allows for the formation of oral ectoderm epithelium on the surface of mouse ES cell aggregates as well as hypothalamic neural tissue in the inner layer adjacent to the oral ectoderm (Fig. 2b). Treatment with a BMP4 antagonist, dorsomorphin, has been shown to suppress the generation of oral ectoderm (Suga et al. 2011). Quantitative polymerase chain reaction analyses revealed significantly higher internal BMP4 expression in LCA aggregates (Suga et al. 2011). Moreover, Koehler et al. succeeded in differentiating the otic placode (Fig. 1a; Koehler et al. 2013), which belongs to the head and oral ectoderm, following BMP treatment of mouse ES cells, which supports the reliability and robustness of this strategy. Our recent study showed that very low concentrations (picomolar level) of exogenous BMP4 treatment facilitated differentiation into non-neural ectoderms, which contained not only pituitary primordium but also dental germs (Ochiai et al. 2015). Taken together, these findings appear to indicate that appropriate BMP4 expression is important for head ectoderm induction (Wilson and Hemmati-Brivanlou 1995; Basch and Bronner-Fraser 2006; Davis and Camper 2007).
Self-Formation of Rathke’s Pouch
In the developing embryo, Rathke’s pouch forms at the midline of the head ectoderm. Shh is expressed in the ventral diencephalon and oral ectoderm but is excluded from the invaginating Rathke’s pouch (Zhu et al. 2007; Wang et al. 2010). Rathke’s pouch receives Shh signals from neighboring tissues in vivo, and Shh is known to provide positional information to adjust towards the midline (Zhu et al. 2007). Therefore, we added smoothened agonist (SAG) as a strong Shh signal to the differentiation medium of mouse ES cell aggregates in vitro. On day 13, multiple oval structures formed in the SAG-treated LCA SFEBq aggregates (Fig. 2c). The vesicles were situated between the oral ectoderm and hypothalamic neurons. Lim3 (formal gene name is Lhx3) expression indicated that the vesicles had similar characteristics to Rathke’s pouch. These Lim3+ tissues appeared as a thick epithelium on the surface that then invaginated and finally formed hollowed vesicles. The length of the major axis was about 200 μm, which is almost equal to the size of the embryonic Rathke’s pouch. The size of Rathke’s pouch seems to be prescribed.
Interactions between oral ectoderm and hypothalamic neurons appear to be critically important. Neither isolated surface ectoderm alone, nor isolated hypothalamic tissues alone, formed Lim3+ pouches. Only in cases where the two divided components were re-assembled did Lim3+ expression recover to some extent (Suga et al. 2011).
These findings demonstrate self-formation of Rathke’s pouch in mouse ES cell aggregates. It has also been shown that Rathke’s pouch forms even without mesenchymal cells, because this model contains only ectodermal cells.
Interestingly, a single aggregate often contains several pouches whereas there is usually only one pouch in the embryo (Suga et al. 2011). This finding suggests that several morphogenetic fields for pituitary placodes can be independently generated within the oral ectoderm epithelium on the surface of the ES cell aggregate, which is reminiscent of the Vax1 knock-out mouse (Bharti et al. 2011). A second Rathke’s pouch develops in addition to the orthotopic anlage in the Vax1 knock-out mouse. Ectopic expression of FGF10, which is expressed in the infundibulum and implicated in pituitary induction, is also detected in the hypothalamic neuroepithelium overlying the second pouch. Thus, Vax1 likely limits the hypothalamic neuroepithelium area that generates pituitary-inducing signals. Indeed, Vax1 expression in vivo is eliminated near the infundibulum, which has inducing activity for pituitary development. In the mouse ES aggregates used for pituitary differentiation in the present study, Vax1-positive cells did not exist in the hypothalamic area. Conversely, Wataya’s aggregate for hypothalamic differentiation (Wataya et al. 2008) has been shown to contain Vax1-positive cells. We speculate that precise positioning in the hypothalamus slightly shifts as a result of BMP4 and Shh signals.
Differentiation Into Hormone-Producing Endocrine Cells
During pituitary development in the embryo, Lim3+ pituitary progenitors commit to several lineages (Davis et al. 2011), i.e., corticotroph, somatotroph, lactotroph, thyrotroph, gonadotroph, and melanotroph lineages. Among them, the ACTH-producing corticotroph lineage expresses the transcription factor Tbx19 prior to ACTH expression. As Notch signaling inhibits Tbx19 expression (Lamolet et al. 2001; Zhu et al. 2006; Kita et al. 2007), we evaluated the effect of the Notch inhibitor DAPT. As a result, DAPT treatment increased Tbx19 expression in SAG-treated LCA SFEBq aggregates. A substantial number of ACTH+ cells appeared in the Tbx19+ lesion (Fig. 2d). Without DAPT treatment, corticotroph differentiation efficiency was decreased, and other lineages were not detected.
Previous reports have shown that canonical Wnt signaling promotes Pit1 expression (DiMattia et al. 1997; Olson et al. 2006; Sornson et al. 1996). Consistent with this finding, treatment with the Wnt agonist BIO increased Pit1 expression, resulting in subsequent GH+ and PRL+ cell differentiation.
Head mesenchyme has been suggested to promote pituitary development in vivo (Gleiberman et al. 1999). Therefore, we applied conditioned medium from PA6 stromal cells to SAG-treated LCA SFEBq aggregates. As a result, we successfully induced luteinizing hormone-positive, follicle-stimulating hormone-positive, and thyroid-stimulating hormone-positive cells. Further investigation is necessary to identify factors in the PA6-conditioned medium.
Lim3 is essential for these hormone-producing lineages. To suppress Lim3 expression in differentiating mouse ES cells, we used the Tet-inducible shRNA expression lentivirus vector system (kindly gifted from Hiroyuki Miyoshi at RIKEN BioResouce Center). Knockdown of Lim3 inhibited subsequent differentiation into hormone-producing cells, which supports altered pituitary development in Lim3 knockout mice (Sheng et al. 1996).
These results demonstrate the competence of ES cell-derived pituitary progenitors to generate multiple endocrine lineages in vitro.
Functionality of Induced ACTH+ Cells
Positive and negative regulations by exogenous stimuli are characteristic for endocrine cells. To investigate in vitro functionality, we induced ACTH+ cells for evaluation because they are most efficiently generated using the SAG-treated LCA SFEBq method.
After 10 min of stimulation by corticotropin releasing hormone (CRH), substantial amounts of ACTH were secreted from SAG-treated LCA SFEBq aggregates in vitro (Fig. 3a). The secreted ACTH concentration was similar to levels in mouse peripheral blood. ACTH secretion from the pituitary gland is negatively regulated by the downstream glucocorticoid hormone. Consistent with this control principle, in vitro ACTH secretion as a result of CRH stimulation was suppressed by glucocorticoid pre-treatment (Fig. 3b).
Functional tests of mouse ES-derived ACTH+ cells. (a) In vitro release from mouse ES-derived ACTH+ cells. F, glucocorticoid pretreatment. Among the releasing factors, CRH most efficiently induces ACTH secretion. (b) Negative feedback test. Pretreatment with hydrocortisone suppresses CRH-stimulated ACTH secretion from aggregates. (c) In vivo functional test by ectopic transplantation. All mice, except for the WT mice, received a hypophysectomy; hypopituitarism was confirmed by CRH loading. S + D+, SAG- and DAPT-treated aggregates. S-D-, no SAG or DAPT treatment. The values shown on graphs represent mean ± s.e.m. *P < 0.05; **P < 0.01; ***P < 0.001 (modified from Suga et al. 2011). (d) Blood ACTH and subsequent release of corticosterone. (e) Improved activity and survival
Similar to in vivo endocrine systems, these data demonstrate that mouse ES cell-derived ACTH+ cells respond to both positive and negative regulators. These hormonal responses to surrounding regulators are indispensable for homeostasis. For this reason, the generation of anterior pituitary tissue that retains regulatory hormonal control in vitro is an important step for the development of cell transplantation therapies for pituitary diseases. Furthermore, we suggest that the endocrine organoid formed in this three-dimensional culture condition might better reflect the in vivo microenvironment. Such approaches may be beneficial for producing other functionally mature endocrine tissues.
Effect of Transplantation Into Hypophysectomized Model Animals
Finally, we evaluated the transplantation effect of the induced ACTH+ cells. Because of technical difficulties, we chose ectopic transplantation into the kidney subcapsule (Fig. 3c) instead of orthotopic transplantation into the sella turcica. At one week after transplantation, blood ACTH levels were slightly, but significantly, increased. CRH loading induced a substantial elevation in blood ACTH levels (Fig. 3d). The downstream glucocorticoid hormone corticosterone was also significantly increased, indicating that ACTH from the graft sufficiently induced the downstream hormone (Fig. 3d).
Even without CRH loading, the basal levels of ACTH were higher. Importantly, corticosterone levels were also increased, suggesting that partial recovery of blood ACTH had a moderate, but biologically significant, effect (note that ED50 of the ACTH receptor MC2R for glucocorticoid production is around 9 pg/mL; Melmed 2011). In accordance with this finding, the treated hypophysectomized mice displayed higher spontaneous locomotor activities and survived significantly longer (Fig. 3e). Although CRH, which is secreted from the hypothalamus, should be diluted in the peripheral site, mouse ES cell derived pituitary tissues rescued survival and spontaneous activities, suggesting that basal secretion from these tissues was sufficient for those effects.
These findings showed that induced ACTH+ cells derived from mouse ES cells acted as endocrine tissues and that regenerative medicine for pituitary dysfunction is feasible.
Adaptation to Human ES/iPS Cell Culture
The recovery of lost pituitary function is an important issue for medical studies because the anterior pituitary has poor potential for regeneration. Because some pituitary dysfunctions cannot be solely treated by drugs (Arima et al. 2014; Hahner et al. 2015; Sherlock et al. 2009), regenerative therapy employing stem cells should be considered as a new form of therapeutic intervention. Our SFEBq method (Suga et al. 2011) induces pituitary cells that can auto-regulate hormonal secretion and respond to changing circumstances. The application of this culture method to human ES cells is necessary for clinical purposes. However, poor survival of human ES cells in SFEB culture might limit the use of these cells for future medical applications. Our colleagues found that a selective Rho-associated kinase (ROCK) inhibitor, Y-27632, markedly diminished dissociation-induced apoptosis of human ES cells and enabled the cells to form aggregates in SFEB culture (Watanabe et al. 2007). Using this fundamentally important discovery, we attempted to adapt our pituitary-differentiating culture method for human ES cell culture. We were able to obtain corticotrophs and somatotrophs from the human ES cells, although these are still preliminary data.
Results demonstrated that the anterior pituitary self-forms in vitro following co-induction of the hypothalamic and oral ectoderm (Fig. 4a). The juxtaposition of these tissues facilitated the formation of the pituitary placode, and their features were consistent with characteristics of Rathke’s pouch in vivo. The human ES cell-derived Rathke’s pouch was much larger than the pouch formed by mouse ES cells, which was in accordance with the size difference between human and mouse embryonic Rathke’s pouches. These pituitary placodes subsequently differentiated into pituitary hormone-producing cells. All six types of pituitary hormone-producing cells were identified (Fig. 4b). Among them, we confirmed that the human ES-derived corticotroph responded normally to releasing and feedback signals. Electron microscopy revealed secretory granules stored in the cytoplasm of these cells (Fig. 4c).
For both mouse and human ES cells, SFEB culture is a favorable method that can generate functional pituitary cells. Future studies will confirm whether human iPS cells can differentiate into pituitary cells using the same culture methods.
Future Perspectives
There are two primary uses for human ES/iPS cell-derived pituitary cells. One is the human model of development or disease. Results from our study showed that the present culture methods recapitulated embryogenesis, suggesting that it could be used in the area of developmental biology. In terms of diseases due to gene mutations, tissues derived from disease-specific iPS cells can be used for therapy screenings in a human disease model.
The second major use for human ES/iPS cell-derived pituitary cells is for regenerative medicine. Although stem cell-based therapeutics provide high expectations for the treatment of diabetes mellitus, the use of regenerative medicine for hypothalamus-hypophyseal dysfunctions has received little attention.
ES cell-derived ACTH-producing cells function even after ectopic transplantation. This finding raises the possibility of relatively simple grafting of artificial ES/iPS cell-derived pituitary tissues into a peripheral site. These cells can function effectively if hormone secretion can be extrinsically controlled by releasing factors or small molecule agonists. However, ectopic transplantation is not perfect because physiological CRH released from the hypothalamus does not directly affect these grafts. Orthotopic transplantation of hormone-producing cells that are controlled by positive and negative regulators is one of the future candidates for complete therapy.
In future studies, it will be challenging to recapitulate an entire anterior pituitary gland that contains all endocrine components in three-dimensional cultures of human ES or iPS cells and to use such artificial pituitary tissues for orthotopic transplantation into the sella of a large mammal. To achieve this long-term goal, further studies are needed before pituitary regenerative medicine can be directly transferred to clinical use.
References
Andoniadou CL, Gaston-Massuet C, Reddy R, Schneider RP, Blasco MA, Le Tissier P, Jacques TS, Pevny LH, Dattani MT, Martinez-Barbera JP (2012) Identification of novel pathways involved in the pathogenesis of human adamantinomatous craniopharyngioma. Acta Neuropathol 124:259–271
Arima H, Wakabayashi T, Nagatani T, Fujii M, Hirakawa A, Murase T, Yambe Y, Yamada T, Yamakawa F, Yamamori I, Yamauchi M, Oiso Y (2014) Adipsia increases risk of death in patients with central diabetes insipidus. Endocr J 61:143–148
Babinet C, Cohen-Tannoudji M (2001) Genome engineering via homologous recombination in mouse embryonic stem (ES) cells: an amazingly versatile tool for the study of mammalian biology. An Acad Bras Cienc 73:365–383
Basch ML, Bronner-Fraser M (2006) Neural crest inducing signals. Adv Exp Med Biol 589:24–31
Ben-Shlomo A (2010) Pituitary gland: predictors of acromegaly-associated mortality. Nat Rev Endocrinol 6:67–69
Bernstein A, Breitman M (1989) Genetic ablation in transgenic mice. Mol Biol Med 6:523–530
Bharti K, Gasper M, Bertuzzi S, Arnheiter H (2011) Lack of the ventral anterior homeodomain transcription factor VAX1 leads to induction of a second pituitary. Development 138:873–878
Brinkmeier ML, Potok MA, Davis SW, Camper SA (2007) TCF4 deficiency expands ventral diencephalon signaling and increases induction of pituitary progenitors. Dev Biol 311:396–407
Chen J, Hersmus N, Van Duppen V, Caesens P, Denef C, Vankelecom H (2005) The adult pituitary contains a cell population displaying stem/progenitor cell and early embryonic characteristics. Endocrinology 146:3985–3998
Chen J, Gremeaux L, Fu Q, Liekens D, Van Laere S, Vankelecom H (2009) Pituitary progenitor cells tracked down by side population dissection. Stem Cells 27:1182–1195
Danjo T, Eiraku M, Muguruma K, Watanabe K, Kawada M, Yanagawa Y, Rubenstein JL, Sasai Y (2011) Subregional specification of embryonic stem cell-derived ventral telencephalic tissues by timed and combinatory treatment with extrinsic signals. J Neurosci 31:1919–1933
Davis SW, Camper SA (2007) Noggin regulates Bmp4 activity during pituitary induction. Dev Biol 305:145–160
Davis SW, Mortensen AH, Camper SA (2011) Birthdating studies reshape models for pituitary gland cell specification. Dev Biol 352:215–227
DiMattia GE, Rhodes SJ, Krones A, Carrière C, O'Connell S, Kalla K, Arias C, Sawchenko P, Rosenfeld MG (1997) The Pit-1 gene is regulated by distinct early and late pituitary-specific enhancers. Dev Biol 182:180–190
Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, Wataya T, Nishiyama A, Muguruma K, Sasai Y (2008) Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3:519–532
Eiraku M, Takata N, Ishibashi H, Kawada M, Sakakura E, Okuda S, Sekiguchi K, Adachi T, Sasai Y (2011) Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472:51–56
Evans MJ, Kaufman MH (1981) Establishment in culture of pluripotential cells from mouse embryos. Nature 292:154–156
Fauquier T, Guérineau NC, McKinney RA, Bauer K, Mollard P (2001) Folliculostellate cell network: a route for long-distance communication in the anterior pituitary. Proc Natl Acad Sci U S A 98:8891–8896
Fu Q, Gremeaux L, Luque RM, Liekens D, Chen J, Buch T, Waisman A, Kineman R, Vankelecom H (2012) The adult pituitary shows stem/progenitor cell activation in response to injury and is capable of regeneration. Endocrinology 153:3224–3235
Garcia-Lavandeira M, Saez C, Diaz-Rodriguez E, Perez-Romero S, Senra A, Dieguez C, Japon MA, Alvarez CV (2012) Craniopharyngiomas express embryonic stem cell markers (SOX2, OCT4, KLF4, and SOX9) as pituitary stem cells but do not coexpress RET/GFRA3 receptors. J Clin Endocrinol Metab 97:E80–87
Gaston-Massuet C, Andoniadou CL, Signore M, Jayakody SA, Charolidi N, Kyeyune R, Vernay B, Jacques TS, Taketo MM, Le Tissier P, Dattani MT, Martinez-Barbera JP (2011) Increased Wingless (Wnt) signaling in pituitary progenitor/stem cells gives rise to pituitary tumors in mice and humans. Proc Natl Acad Sci U S A 108:11482–11487
Gleiberman AS, Fedtsova NG, Rosenfeld MG (1999) Tissue interactions in the induction of anterior pituitary: role of the ventral diencephalon, mesenchyme, and notochord. Dev Biol 213:340–353
Gremeaux L, Fu Q, Chen J, Vankelecom H (2012) Activated phenotype of the pituitary stem/progenitor cell compartment during the early-postnatal maturation phase of the gland. Stem Cells Dev 21:801–813
Hahner S, Spinnler C, Fassnacht M, Burger-Stritt S, Lang K, Milovanovic D, Beuschlein F, Willenberg HS, Quinkler M, Allolio B (2015) High incidence of adrenal crisis in educated patients with chronic adrenal insufficiency: a prospective study. J Clin Endocrinol Metab 100:407–416
Ikeda H, Osakada F, Watanabe K, Mizuseki K, Haraguchi T, Miyoshi H, Kamiya D, Honda Y, Sasai N, Yoshimura N, Takahashi M, Sasai Y (2005) Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. Proc Natl Acad Sci U S A 102:11331–11336
Kadoshima T, Sakaguchi H, Nakano T, Soen M, Ando S, Eiraku M, Sasai Y (2013) Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc Natl Acad Sci U S A 110:20284–20289
Kawasaki H, Suemori H, Mizuseki K, Watanabe K, Urano F, Ichinose H, Haruta M, Takahashi M, Yoshikawa K, Nishikawa S, Nakatsuji N, Sasai Y (2002) Generation of dopaminergic neurons and pigmented epithelia from primate ES cells by stromal cell-derived inducing activity. Proc Natl Acad Sci U S A 99:1580–1585
Kikuchi M, Yatabe M, Kouki T, Fujiwara K, Takigami S, Sakamoto A, Yashiro T (2007) Changes in E- and N-cadherin expression in developing rat adenohypophysis. Anat Rec 290:486–490
Kita A, Imayoshi I, Hojo M, Kitagawa M, Kokubu H, Ohsawa R, Ohtsuka T, Kageyama R, Hashimoto N (2007) Hes1 and Hes5 control the progenitor pool, intermediate lobe specification, and posterior lobe formation in the pituitary development. Mol Endocrinol 21:1458–1466
Koehler KR, Mikosz AM, Molosh AI, Patel D, Hashino E (2013) Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture. Nature 500:217–221
Lamolet B, Pulichino AM, Lamonerie T, Gauthier Y, Brue T, Enjalbert A, Drouin J (2001) A pituitary cell-restricted T box factor, Tpit, activates POMC transcription in cooperation with Pitx homeoproteins. Cell 104:849–859
Langlais D, Couture C, Kmita M, Drouin J (2013) Adult pituitary cell maintenance: lineage-specific contribution of self-duplication. Mol Endocrinol 27:1103–1112
Li H, Collado M, Villasante A, Matheu A, Lynch CJ, Cañamero M, Rizzoti K, Carneiro C, Martínez G, Vidal A, Lovell-Badge R, Serrano M (2012) p27(Kip1) directly represses Sox2 during embryonic stem cell differentiation. Cell Stem Cell 11:845–852
Luque RM, Lin Q, Córdoba-Chacón J, Subbaiah PV, Buch T, Waisman A, Vankelecom H, Kineman RD (2011) Metabolic impact of adult-onset, isolated, growth hormone deficiency (AOiGHD) due to destruction of pituitary somatotropes. PLoS One 6, e15767
Melmed S (ed) (2011) The pituitary, 3rd edn. Academic Press, Cambridge, MA, p 61
Mollard P, Hodson DJ, Lafont C, Rizzoti K, Drouin J (2012) A tridimensional view of pituitary development and function. Trends Endocrinol Metab 23:261–269
Morizane A, Takahashi J, Shinoyama M, Ideguchi M, Takagi Y, Fukuda H, Koyanagi M, Sasai Y, Hashimoto N (2006) Generation of graftable dopaminergic neuron progenitors from mouse ES cells by a combination of coculture and neurosphere methods. J Neurosci Res 83:1015–1027
Muguruma K, Nishiyama A, Ono Y, Miyawaki H, Mizuhara E, Hori S, Kakizuka A, Obata K, Yanagawa Y, Hirano T, Sasai Y (2010) Ontogeny-recapitulating generation and tissue integration of ES cell-derived Purkinje cells. Nat Neurosci 13:1171–1180
Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, Saito K, Yonemura S, Eiraku M, Sasai Y (2012) Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10:771–785
Ochiai H, Suga H, Yamada T, Sakakibara M, Kasai T, Ozone C, Ogawa K, Goto M, Banno R, Tsunekawa S, Sugimura Y, Arima H, Oiso Y (2015) BMP4 and FGF strongly induce differentiation of mouse ES cells into oral ectoderm. Stem Cell Res 15:290–298
Olson LE, Tollkuhn J, Scafoglio C, Krones A, Zhang J, Ohgi KA, Wu W, Taketo MM, Kemler R, Grosschedl R, Rose D, Li X, Rosenfeld MG (2006) Homeodomain-mediated beta-catenin-dependent switching events dictate cell-lineage determination. Cell 125:593–605
Osakada F, Ikeda H, Mandai M, Wataya T, Watanabe K, Yoshimura N, Akaike A, Sasai Y, Takahashi M (2008) Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotechnol 26:215–224
Sakaguchi H, Kadoshima T, Soen M, Narii N, Ishida Y, Ohgushi M, Takahashi J, Eiraku M, Sasai Y (2015) Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat Commun 6:8896
Sasai Y, Eiraku M, Suga H (2012) In vitro organogenesis in three dimensions: self-organising stem cells. Development 139:4111–4121
Sheng HZ, Zhadanov AB, Mosinger B Jr, Fujii T, Bertuzzi S, Grinberg A, Lee EJ, Huang SP, Mahon KA, Westphal H (1996) Specification of pituitary cell lineages by the LIM homeobox gene Lhx3. Science 272:1004–1007
Sherlock M, Reulen RC, Alonso AA, Ayuk J, Clayton RN, Sheppard MC, Hawkins MM, Bates AS, Stewart PM (2009) ACTH deficiency, higher doses of hydrocortisone replacement, and radiotherapy are independent predictors of mortality in patients with acromegaly. J Clin Endocrinol Metab 94:4216–4223
Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O'Connell SM, Gukovsky I, Carrière C, Ryan AK, Miller AP, Zuo L, Gleiberman AS, Andersen B, Beamer WG, Rosenfeld MG (1996) Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature 384:327–333
Suga H, Kadoshima T, Minaguchi M, Ohgushi M, Soen M, Nakano T, Takata N, Wataya T, Muguruma K, Miyoshi H, Yonemura S, Oiso Y, Sasai Y (2011) Self-formation of functional adenohypophysis in three-dimensional culture. Nature 480:57–62
Takuma N, Sheng HZ, Furuta Y, Ward JM, Sharma K, Hogan BL, Pfaff SL, Westphal H, Kimura S, Mahon KA (1998) Formation of Rathke's pouch requires dual induction from the diencephalon. Development 125:4835–4840
Wang Y, Martin JF, Bai CB (2010) Direct and indirect requirements of Shh/Gli signaling in early pituitary development. Dev Biol 348:199–209
Watanabe K, Kamiya D, Nishiyama A, Katayama T, Nozaki S, Kawasaki H, Watanabe Y, Mizuseki K, Sasai Y (2005) Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci 8:288–296
Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, Sasai Y (2007) A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol 25:681–686
Wataya T, Ando S, Muguruma K, Ikeda H, Watanabe K, Eiraku M, Kawada M, Takahashi J, Hashimoto N, Sasai Y (2008) Minimization of exogenous signals in ES cell culture induces rostral hypothalamic differentiation. Proc Natl Acad Sci USA 105:11796–11801
Wilson PA, Hemmati-Brivanlou A (1995) Induction of epidermis and inhibition of neural fate by Bmp-4. Nature 376:331–333
Zhu X, Zhang J, Tollkuhn J, Ohsawa R, Bresnick EH, Guillemot F, Kageyama R, Rosenfeld MG (2006) Sustained Notch signaling in progenitors is required for sequential emergence of distinct cell lineages during organogenesis. Genes Dev 20:2739–2753
Zhu X, Gleiberman AS, Rosenfeld MG (2007) Molecular physiology of pituitary development: signaling and transcriptional networks. Physiol Rev 87:933–963
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, a link is provided to the Creative Commons license and any changes made are indicated.
The images or other third party material in this chapter are included in the work's Creative Commons license, unless indicated otherwise in the credit line; if such material is not included in the work's Creative Commons license and the respective action is not permitted by statutory regulation, users will need to obtain permission from the license holder to duplicate, adapt or reproduce the material.
Copyright information
© 2016 The Author(s)
About this chapter
Cite this chapter
Suga, H. (2016). Recapitulating Hypothalamus and Pituitary Development Using Embryonic Stem/Induced Pluripotent Stem Cells. In: Pfaff, D., Christen, Y. (eds) Stem Cells in Neuroendocrinology. Research and Perspectives in Endocrine Interactions. Springer, Cham. https://doi.org/10.1007/978-3-319-41603-8_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-41603-8_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-41602-1
Online ISBN: 978-3-319-41603-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)