Abstract
The replacement of damaged or lost tissue secondary to injury or disease is fast becoming a popular concept in twenty-first century medicine. The regeneration or replacement of tissue encompasses the idea of return of original function and morphology of an injured tissue via the process of self or aided healing. The majority of tissues in the human body do not naturally regenerate as their cell lineages have already differentiated and matured. They cannot habitually break down, de-differentiate and replace the damaged tissue.
Increasingly research turns to the notion of integrating stem cells with the principles of tissue engineering to aid surgical practice manage damaged or lost tissue. Over the last decade or so substantial efforts have been made to drive this research forward and convert its outcomes into clinical practice.
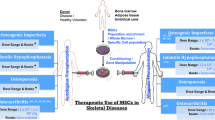
Multipotent mesenchymal stromal cells (MSCs) are the most common type of stem cell used where the regeneration of skeletal tissue is concerned. To date a vast majority of the research that has been undertaken in this field has been preclinical with limited data from randomised controlled trials. Variation in research outcomes has also let controversy in the role of stem cell therapy and tissue engineering techniques in certain pathologies. However the potential of MSCs as an established treatment for skeletal tissue regeneration is undeniable, even if they are still fraught by their pitfalls in translational research. Knowledge of MSCs biology has developed and prospered significantly since they were first discovered and this has been critical in its development in skeletal tissue regeneration.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- CFU-F:
-
Fibroblastic colony forming units
- MHC1:
-
Major histocompatibility 1
- MSC:
-
Mesenchymal stem cells
- NKC:
-
Natural killer cell
- VEGF:
-
Vascular endothelial growth factor
References
Afanasyev BV, Elstner EE, Zander AR. A. J. Freidenstein, founder of the mesenchymal stem cell concept. Cell Ther Transplant. 2009;1(3):35–8.
Ardakani AG, Cheema U, Brown RA, Shipley RJ. Quantifying the correlation between spatially defined oxygen gradients and cell fate in an engineered three-dimensional culture model. J R Soc Interface. 2014;11:20140501.
Arthur A, Zannettino A, Gronthos S. The therapeutic applications of multipotential mesenchymal/stromal stem cells in skeletal tissue repair. J Cell Physiol. 2009;218(2):237–45.
Awad HA, Butler DL, Boivin GP. Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng. 2007;5(3):267–77.
Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25(6):1384–92.
Bielby R, Jones E, McGonagle D. The role of mesenchymal stem cells in maintenance and repair of bone. Injury. 2007;38(1):S26–32.
Brown RG, Wiseman M, Chuo CB, Cheema U, Nazhat SG. Ultrarapid engineering of biomimetic materials and tissues: fabrication of nano- and microstructures by plastic compression. Adv Funct Mater. 2005;15(11):1762–70.
Caplan AI, Bruder SP. Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med. 2001;7(6):259–64.
Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076–84.
Chatterjea A, Meijer G, van Blitterswijk C, de Boer J. Clinical application of human mesenchymal stromal cells for bone tissue engineering. Stem Cell Int. 2010;2010:215625.
Chen FH, Tuan RS. Mesenchymal stem cells in arthritic diseases. Arthritis Res Ther. 2008;10(5):223.
Chen FH, Rousche KT, Tuan RS. Technology insight: adult stem cells in cartilage regeneration and tissue engineering. Nat Clin Pract Rheumatol. 2006;2(7):373–82.
Chen Y, Shao JZ, Xiang LX, Dong XJ, Zhang GR. Mesenchymal stem cells: a promising candidate in regenerative medicine. Int J Biochem Cell Biol. 2008;40(5):815–20.
Chimutengwende-Gordon M, Khan W, Haddad B. Editorial: advances and controversies in stem cell therapies and tissue engineering strategies applicable to trauma and orthopaedic surgery. Curr Stem Cell Res Ther. 2013;8(6):415–7.
Clinicaltrials.gov. U.S. National Institute of Health. 2011. http://clinicaltrials.gov/ct2/results?term=mesenchymal+stem+cells+.
Crowley C, Wong JM, Fisher D, Khan WS. A systematic review on preclinical and clinical studies on the use of scaffolds for bone repair in skeletal defects. Curr Stem Cell Res Ther. 2013;8(3):243–52.
Da Silva ML, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(Pt 11):2204–13.
Dhinsa BS, Mahapatra AN, Khan WS. Sources of adult mesenchymal stem cells for ligament and tendon tissue engineering. Curr Stem Cell Res Ther. 2015;10(1):26–30.
Diekman BO, Wu CL, Louer CR, Furman BD. Intra-articular de- livery of purified mesenchymal stem cells from C57BL/6 or MRL/MpJ superhealer mice prevents post-traumatic arthritis. Cell Transplant. 2013;22(8):1395.
Dimitriou R, Jones E, McGonagle D, Giannoudis PV. Bone regeneration: current concepts and future directions. BMC Med. 2011;9:66.
Djouad F, Mrugala D, Noel D, Jorgensen C. Engineered mesenchymal stem cells for cartilage repair. Regen Med. 2006;1(4):529–37.
Djouad F, Bouffi C, Ghannam S, Noel D, Jorgensen C. Mesenchymal stem cells: innovative therapeutic tools for rheumatic diseases. Nat Rev Rheumatol. 2009;5(7):392–9.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, Deans RJ, Keating A, Prockop DJ, Horwitz EM. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–7.
Dudics V, Kunstar A, Kovacs J, Lakatos T, Geher P, Gomor B, Monostori E, Uher F. Chondrogenic potential of mesenchymal stem cells from patients with rheumatoid arthritis and osteoarthritis: measurements in a microculture system. Cells Tissues Organs. 2009;189(5):307–16.
Fiers W, Beyaert R, Brouckaert P, Everaerdt B, Haegeman G, Suffys P, Tavernier J, Vandenabeele P, Vanhaesebroeck B, Vanostade X, Vanroy F. Gene cloning and structure – function relationship of cytokines such As Tnf and interleukins. Immunol Lett. 1987;16(3–4):219–26.
Fisher DM, Wong JM, Crowley C, Khan WS. Preclinical and clinical studies on the use of growth factors for bone repair: a systematic review. Curr Stem Cell Res Ther. 2013;8(3):260–8.
Friedens AJ, Chailakh RK, Lalykina KS. Development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3(4):393.
Giannoudis PV, Pountos I. Tissue regeneration: the past, the present and the future. Injury. 2005;36(4):S2–5.
Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100(9):1249–60.
Hampson K, Forsyth NR, El Haj A, Maffulli N. Tendon tissue engineering. Top Tissue Eng. 2008;4:1–21.
Hoffmann A, Pelled G, Turgeman G, et al. Neotendon formation induced by manipulation of the Smad8 signalling pathway in mes-enchymal stem cells. J Clin Invest. 2006;116(4):940–52.
Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A. Clarification of the nomenclature for MSC: the international society for cellular therapy position statement. Cytotherapy. 2005;7(5):393–5.
Ivkovic A, Marijanovic I, Hudetz D, et al. Regenerative medicine and tissue engineering in orthopaedic surgery. Front Biosci (Elite Ed). 2011;3:923–44.
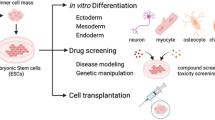
Javazon EH, Beggs KJ, Flake AW. Mesenchymal stem cells: paradoxes of passaging. Exp Hematol. 2004;32(5):414–25.
Jones E, Yang XB. Mesenchymal stem cells and bone regeneration: current status. Injury Int J Care Injured. 2011;42(6):562–8.
Jones S, Horwood N, Cope A, Dazzzi F. The antiproliferative effect of mesenchymal stem cells is a fundamental property shared by all stromal cells. J Immunol. 2007;179(5):2824–31.
Ju SH, Teng GJ, Lu HH, Jin JY, Zhang Y, Zhang AF, Ni YC. In vivo differentiation of magnetically labeled mesenchymal stem cells into hepatocytes for cell therapy to repair damaged liver. Investig Radiol. 2010;45(10):625–33.
Kanczler JA, Ginty PJ, Barry JJA, Clarke NMP, Howdle SM, Shakesheff KM, Oreffo ROC. The effect of mesenchymal populations and vascular endothelial growth factor delivered from biodegradable polymer scaffolds on bone formation. Biomaterials. 2008;29(12):1892–900.
Kanitkar M, Tailor HD, Khan WS. The use of growth factors and mesenchymal stem cells in orthopaedics. Open Orthop J. 2011;5(2):268–74.
Kasper G, Dankert N, Tuischer J, Hoeft M, Gaber T, Glaeser JD, Zander D, Tschirschmann M, Thompson M, Matziolis G, Duda GN. Mesenchymal stem cells regulate angiogenesis according to their mechanical environment. Stem Cells. 2007;25(4):903–10.
Khan WS, Hardingham TE. The characterisation of mesenchymal stem cells: a stem cell is not a stem cell is not a stem cell. J Stem Cells. 2012a;7(2):87–95.
Khan WS, Hardingham TE. Mesenchymal stem cells, sources of cells and differentiation potential. J Stem Cells. 2012b;7(2):75–85.
Khan WS, Longo UG, Adesida A, Denaro V. Stem cell and tissue engineering applications in orthopaedics and musculoskeletal medicine. Stem Cell Int. 2012. Article ID 403170.
Koide Y, Morikawa S, Mabuchi Y, Muguruma Y, Hiratsu E, Hasegawa K, Kobayashi M, Ando K, Kinjo K, Okano H, Matsuzaki Y. Two distinct stem cell lineages in murine bone marrow. Stem Cells. 2007;25(5):1213–21.
Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96(19):10711–6.
Le Blanc K, Gotherstrom C, Ringden O, Hassan M, McMahon R, Horwitz E, Anneren G, Axelsson O, Nunn J, Ewald U, Norden-Lindeberg S, Jansson M, Dalton A, Astrom E, Westgren M. Fetal mesenchymal stem-cell engraftment in bone after in utero transplantation in a patient with severe osteogenesis imperfecta. Transplantation. 2005;79(11):1607–14.
Lee EH, Hui JH. The potential of stem cells in orthopaedic surgery. J Bone Joint Surg (Br). 2006;88:841–51.
Li LL, Zhang Y, Yu B. Mesenchymal stem cell transplantation attenuates cardiac fibrosis associated with isoproterenol-induced global heart failure. Circulation. 2010;122(2):E316.
Longo UG, Rizzello G, Berton A, Ciuffreda M, Migliorini F, Khan WS, Denaro V. Potential of adipose derived stem cells in orthopaedic surgery. Curr Stem Cell Res Ther. 2013;8(6):418–21.
Mankani MH, Kuznetsov SA, Robey PG. Formation of hematopoietic territories and bone by transplanted human bone marrow stromal cells requires a critical cell density. Exp Hematol. 2007;35(6):995–1004.
Mudera V, Morgan M, Cheema U, Nazhat S, Brown R. Ultra-rapid engineered collagen constructs tested in an in vivo nursery site. J Tissue Eng Regen Med. 2007;1(3):192–8.
Muller I, Vaegler M, Holzwarth C, Tzaribatchev N, Pfister SM, Schuett B, Reize P, Greil J, Handgretinger R, Rudert M. Secretion of angiogenic proteins by human multipotent mesenchymal stromal cells and their clinical potential in the treatment of avascular osteonecrosis. Leukemia. 2008;22(11):2054–61.
Nemeth K, Leelahavanichkul A, Yuen PST, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu XZ, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42–9.
O’Donoghue K, Chan J, de la Fuente J, Kennea N, Sandison A, Anderson JR, Roberts IAG, Fisk NM. Microchimerism in female bone marrow and bone decades after fetal mesenchymal stem-cell trafficking in pregnancy. Lancet. 2004;364(9429):179–82.
Ouyang HW, Goh J, Lee EH. Use of bone marrow stromal cells for tendon graft-to-bone healing histological and immunohisto-chemical studies in a Rabbit model. Am J Sports Med. 2004;32(2):321–7.
Pastides PS, Khan WS. Cell-based therapies in musculoskeletal injuries: the evolving role of bone marrow-derived mesenchymal stem cells. Br J Med Med Res. 2011;1(4):486–500.
Patel SA, Meyer JR, Greco SJ, Corcoran KE, Bryan M, Rameshwar P. Mesenchymal stem cells protect breast cancer cells through regulatory t cells: role of mesenchymal stem cell-derived TGF-beta. J Immunol. 2010;184(10):5885–94.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7.
Quarto R, Mastrogiacomo M, Cancedda R. Repair of large bone defects with the use of autologous bone marrow stromal cells. N Engl J Med. 2001;344(5):385–6.
Raheja LF, Galuppo LD, Bowers-Lepore J. Treatment of bilateral medial femoral condyle articular cartilage fissures in a horse using bone marrow-derived multipotent mesenchymal stromal cells. J Equine Vet Sci. 2011;31(3):147–54.
Ramasamy R, Lam EWF, Soeiro I, Tisato V, Bonnet D, Dazzi F. Mesenchymal stem cells inhibit proliferation and apoptosis of tumor cells: impact on in vivo tumor growth. Leukemia. 2007;21(2):304–10.
Schaffler A, Buchler C. Concise review: adipose tissue-derived stromal cells–basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–27
Sethe S, Scutt A, Stolzing A. Aging of mesenchymal stem cells. Ageing Res Rev. 2006;5(1):91–116.
Singh J, Onimowo J, Khan WS. Bone marrow derived stem cells in trauma and orthopaedics: a review of the current trend. Curr Stem Cell Res Ther. 2015;10(1):37–42.
Smith JO, Aarvold A, Tayton ER, Dunlop DG, Oreffo ROC. Skeletal tissue regeneration: current approaches, challenges, and novel reconstructive strategies for an aging population. Tissue Eng B Rev. 2011;17(5):307–20.
Stock UA, Vacanti JP. Tissue engineering: current state and prospects. Annu Rev Med. 2001;52:443–51.
Stocum DL. Regenerative biology and medicine. J Musculoskelet Neuronal Interact. 2002;2(3):270–3.
Strebhardt K, Ullrich A. Paul Ehrlich’s magic bullet concept: 100 years of progress. Nat Rev Cancer. 2008;8(6):473–80.
Tang YL, Zhao Q, Zhang YC, Cheng LL, Liu MY, Shi JH, Yang YZ, Pan CZ, Ge JB, Phillips MI. Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept. 2004;117(1):3–10.
Thanabalasundaram G, Arumalla N, Tailor HD, Khan WS. Regulation of differentiation of mesenchymal stem cells into musculoskeletal cells. Curr Stem Cell Res Ther. 2012;7(2):95–102.
Tucker BA, Karamsadkar SS, Khan WS, Pastides P. The role of bone marrow derived mesenchymal stem cells in sports injuries. J Stem Cells. 2010;5(4):155–66.
Vaananen HK. Mesenchymal stem cells. Ann Med. 2005;37(7):469–79.
Wong RSY. Mesenchymal stem cells: angels or demons? J Biomed Biotechnol. 2011. doi:10.1155/2011/459510.
Yi L, Chen JL, Zhang CL, Wang L, Lu DY, Katakowski M, Gao Q, Shen LH, Zhang J, Lu M, Chopp M. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49(3):407–17.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Ardakani, A., Khan, W.S. (2016). Mesenchymal Stem Cells: Are They the Magic Bullet for Skeletal Tissue Regeneration?. In: Pham, P. (eds) Bone and Cartilage Regeneration. Stem Cells in Clinical Applications. Springer, Cham. https://doi.org/10.1007/978-3-319-40144-7_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-40144-7_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-40143-0
Online ISBN: 978-3-319-40144-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)