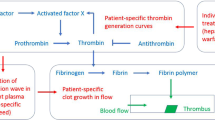
Abstract
This chapter presents an overview and introduction to blood coagulation models. The historical exposure of the development of classical coagulation modeling theories is followed by a basic overview of blood coagulation biochemistry. The recent developments of cell-based models are explained in detail to demonstrate the current shift from the classical cascade/waterfall models. This phenomenological overview is followed by a survey of available mathematical concepts used to describe the blood coagulation process at various spatial scales including some of the related biophysical phenomena. A comprehensive survey of basic literature is provided for each of these topics.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Notes
- 1.
Pregnancy may increase the risk of thrombosis in various ways. The swollen uterus can compress pelvic vessels, reducing blood circulation in the legs. Hormonal changes can also produce hypercoagulability by increasing the concentration in blood of pro-coagulant factors and reducing the concentration of anticoagulant factors. A more serious condition sometimes related to pregnancy is the Antiphospholipid Antibody (or sticky blood) Syndrome, due to the autoimmune production of antibodies against a cell membrane substance called phospholipid causing platelets aggregation.
- 2.
The most famous physician of ancient Egypt was Imhotep (a semi-divine character, he lived during the twenty-seventh century bc and is supposed to be the legendary author of this papyrus). The practice of mummifying corpses must have taught much to Egyptians about the human body, but the papyrus (mainly dealing with wounds healing) can hardly be considered a scientific document and the suggested remedies could easily be fatal to the patients because they could produce infections.
- 3.
He was the author of the humoral theory, according to which four humors (blood, phlegm, black bile, and yellow bile) had to be in a proper balance in healthy individuals. The theory, somehow anticipated by Alcmaeon of Croton (fifth century bc), parallels the contemporary claim by Empedocles that four elements (air, water, fire, earth) are the basic constituents of the world and which may have had a much older origin. Hippocrates tremendous authority (and the immense reputation of Galen, who took his legacy to the Roman world and passed it on to the next era) prevented the development of medicine on a scientific basis for centuries, thanks to the blindness of his followers. The humoral theory found its way through Islamic medicine: the Persian Avicenna (Ib Sı̄nā, 980–1037) based his Canon of Medicine (1025) on Hippocrates’ and Galen’s theories. It was instead opposed by another Persian, Razi (Muhammad ibn Zakariyā Rāzı̄, 865–925) an eclectic scientist, very famous in his times, who explicitly questioned several of Galen claims on the basis of his own experimental observations.
- 4.
He adopted and propagated Hippocrate humoral theory, adding his own theory of four temperaments (choleric, melancholic, sanguine, phlegmatic), resulting from the combinations of the humors with four qualities of (cold, warm, moisty, dry). He sketched an erroneous scheme of the circulatory system. We had to wait until the famous treatise Exercitatio anatomica de motu cordis et sanguinis in animalibus (1628) by William Harvey (1578–1657) for a correct systematic description of blood circulation (limited to great vessels: microcirculation was a later discovery). It is worth mentioning at this point the important contributions later given by the English eclectic scientist Stephen Hales (1677–1761), who determined the blood volume, the heart output and who first measured arterial blood pressure. For completeness we recall the revolutionary work of Andreas Vesalius (Latinized from Andries van Wesel) (1514–1564), who opened a new era in physiology. It is interesting to note that Vesalius studied Rāzı̄’s books and that he based his famous treatise De humani corporis fabrica libri septem (1543) on direct observation of dissected human bodies. He pointed out several of Galen’s mistakes (particularly in the description of circulatory system), indifferent to the harsh criticism of Galen’s followers.
- 5.
Explanations given to pregnancy or post-partum related limb swelling by various authors during the seventeenth and eighteenth centuries, largely based on humors look today simply ridiculous. See the paper [13].
- 6.
Such a fibrous component was isolated much later by Marcello Malpighi (1628–1694), the Italian physician famous above all for his studies on kidneys.
- 7.
A new course in the medical studies was set by the book The Philosophical Principles of Medicine (1725) by Thomas Morgan.
- 8.
Cells were first observed at the microscope by the physicist Robert Hooke (1635–1703) (the founder of the theory of elasticity) in a thin sample of cork (1665). He did not know what cells were, but he called them that way because of their particular and regular arrangement in the sample, resembling the one of monks cells.
- 9.
Malpighi first described RBC as fat corpuscles (1663). Actually RBCs had been observed earlier (1658) by the Dutch Jan Swammerdam (1637–1680). Malpighi was also the discoverer of capillaries (1661).
- 10.
It is really amazing that a well-known scientist like the Swedish Robin Fåhraeus (1888–1968), still quoted today, for instance, in the field of blood rheology, believed to have found a confirmation of Galens theories on the basis of the observation that blood coagulates in four layers with different colours, corresponding to the famous humors [121].
- 11.
Virchow described the mechanism of thromboembolism [240], a phenomenon that was by no means clear at his time (inflammation was considered by many physicians the real cause of thrombosis: this was the subject of a famous dispute with the French pathologist Jean Cruveilhier). Curiously, he did not formulate the famous triad which for some reason found a firm place in the literature much after his death (apparently not before 1950!). See the interesting review [17].
- 12.
Circumcision is a very old practice, already found in the ancient Egypt and that was widely adopted also in the Islamic world. Its origin in ancient Egypt was probably as an initiation practice to religious offices. The Book of the Dead describes self-circumcision by the sun-god Ra: Blood fell from the phallas of Ra after he had finished cutting himself.
- 13.
In numbers, 1 over 10,000 men is hemophilic. The probability that a woman is hemophilic is the square of that number.
- 14.
In this connection the name of the eminent French hematologist Georges Hayem (1841–1933) has to be remembered as one of the founders of modern hematology. He performed the first count of platelets. In 1882 he illustrated the effects of thrombocytopenia (low platelets count).
- 15.
Large molecules like proteins have specific sites which are engaged in specific reactions.
- 16.
All data concerning human blood are subjected to large variations, according to sex, body weight, and health conditions.
- 17.
AMP, ADP, ATP contain 1 (Mono-), 2 (Di-), 3 (Tri-) atoms of phosphorus and they are obtained in that sequence by addition of a P atom (a process called phosphorylation). ATP has a vital importance in cells metabolism.
- 18.
This condition can be produced by arterial stenosis, possibly as a consequence of clotting itself, or due to the mechanical action of implanted devices (rigid artificial heart valves).
- 19.
Platelet Factors 1–3 actually regulate interactions with the Coagulation Factors IIa (thrombin), V, X.
- 20.
Ecto-enzymes act at the exterior of cells. For more details about this enzyme see, [87].
- 21.
Though there is some disagreement on the exact meaning of this name, it is very frequently attributed to TF.
- 22.
Serine proteases are a large class of enzymes including the amino acid serine. The list of serine proteases is impressively long. See http://biochem.wustl.edu/~protease/ser_pro_help.html.
- 23.
Simultaneous and independent discoveries had produced a great confusion in nomenclature.
- 24.
Actually there were many more names: see [208] for a complete list.
- 25.
Fibrinogen is not just the precursor of Fibrin, but it has also other specific functions, illustrated in this chapter. It is also known to stimulate RBCs aggregation (forming the so-called rouleaux), a phenomenon of some importance in blood rheology.
- 26.
In order to prevent coagulation and keep blood flowing through the wound it produces, the Hirudo Medicinalis (leech) secretes Hirudin, a natural and very effective inhibitor of thrombin.
- 27.
Stephen Christmas was the first patient diagnosed with FIX deficiency (hemophilia B) (1952) at the age of five. He died in 1993 by AIDS. Many of the transfusion-dependent patients were infected by the HIV virus before blood screening became obligatory. A case which became emblematic was the one of Ryan Wayne White, affected by hemophilia A, who became discriminated when he was diagnosed with AIDS. He died still a teenager in 1990.
- 28.
Named after the patients Rufus Stuart and Audrey Prower.
- 29.
Named after Ratnoff’s patient John Hageman (1955).
- 30.
Laki and Lorand suggested its existence in 1948 [138].
- 31.
A very important function of Protein S in the organism is to facilitate phagocytosis of apoptotic cells by macrophages. Discovered in 1979 in Seattle, takes its name after that city.
- 32.
Denominated after the German name Koagulationvitamin. Discovered in the 1930 a Nobel prize was attributed in 1943 for studies on it, though its real action in the coagulation process became clear only in the 1970.
- 33.
Patented in 1948 as a rat poison and used as anticoagulant for humans since 1954. It was isolated in 1941 by a group at the University of Wisconsin after a 6-year work investigating a widespread hemorrhagic disease that affected cattle in the USA, the so-called sweet clover disease (the research was funded by WARF, i.e., Wisconsin Alumni Research Foundation). See [248].
- 34.
Already in the 1950 it was known that deficiency of vWF was accompanied by a deficiency of FVIII (see [208]).
- 35.
The symbol X-ase is sometimes used.
- 36.
Also referred to as Williams Factor or Flaujeac Factor.
- 37.
Kininogens are proteins which are precursors of kinins (see next footnote), such as bradikinin and kallidin, which are vasodilator.
- 38.
Here we refer to Plasma Kallikrein, distinct from the numerous group of Tissue Kallikreins, which are enzymes performing various actions. Discovered in 1934, it was named after the Greek words kalli (sweet, in this context) and krein (flesh) referring to pancreas tissue. Plasma Kallikrein (like some of its tissue analogs) liberates kinins from the kininogens. The so-called kinin-kallikrein system has a role in regulating blood pressure, owing to the vasodilation action.
- 39.
First isolated in the urine.
- 40.
Also Kallikrein and FXIIa can activate plasminogen.
- 41.
AT I–IV are also found in the literature, with specific targets.
- 42.
Discovered in 1918 [109], though isolated in 1916 in canine liver tissue [167]. There has been some controversy about heparin discovery (see [248] and [162]]). It is a large polymer, also naturally produced by endothelial cells (as heparan sulfate). A side effect can be a strong reduction of platelets count (Heparin Induced Thrombocytopenia, HIT), see [128]. HIT can be sometimes observed in patients undergoing hemodialysis, during which heparin is supplied to prevent clotting (after passing through the dialyzer and before being returned to the patient, protamine sulfate is added, which neutralizes heparin’s action). Platelet Factor 4 contrasts the action of heparin on platelets.
- 43.
Its deficiency leads to degradation of tissues, particularly in the lungs, causing emphysema. Smoke is believed to inactivate this serpin, thus causing additional damage to lungs.
- 44.
PAI2 is detectable only in pregnant women, a fact that may justify the increased risk of thrombosis during pregnancy.
- 45.
Some FVIIa can reach TF in nonvascular tissues even in the absence of a lesion [279], thus making FIXa and FXa accidentally available. However, coagulation does not start because it requires, for instance, the intervention of platelets, which are not available out of the bloodstream.
- 46.
The proteins responsible for this regulatory action have a fundamental role in eventually halting the clot growth. They are inevitably produced at this initial stage too, but it is known that FVa inhibition by APC is far less efficient than on the surface of endothelial cells [106, 219]. One can wonder whether, besides the clotting confining action, the simultaneous slowing down of the initial process may have a precise aim, for instance letting the platelet plug become thicker.
- 47.
The Latin word for purple. Purpura denotes spots in the range 3–10 mm, smaller spots are called petechiae, and those more extended are called ecchymoses.
- 48.
The Latin equivalent of the Greek derived word idiopathic is sui generis. In this context it means of no specific origin.
- 49.
Congenital deficiency accounts for a small fraction of TTP cases and is known as Upshaw–Schülman syndrome.
- 50.
More common among Ashkenazi Jews.
- 51.
Because immobilization is a frequent cause, DVT is also called the economy class syndrome, since many cases have been reported in passengers after long flights.
- 52.
In that case it is known as Paget–Schrötter disease.
- 53.
Not with fibrinolytic proteins (like tPA or UPA), because they could fragment rather than gradually dissolve the clot. Fibrinolytic therapies are instead used to attack arterial thrombosis in the heart or the brain.
- 54.
Major veins are provided with valves preventing flux inversion, thus helping circulation in the presence of reduced pressure gradients.
- 55.
Reduced oxygen concentration is more marked in valves, since, differently from veins and other blood vessel, they do not possess their own vessels (vasa vasorum).
- 56.
This paper is an extensive study on the role of TF and of thrombin in promoting angiogenesis and contains a large bibliography. Excessive TF production may be accompanied by upregulated expression of VEGF (the angiogenic factor) and downregulated expression of thrombospondin 1 (see Sect. 7.3).
- 57.
Clots are mostly originated in the left atrium and more precisely in an area called left atrial appendage.
- 58.
- 59.
The scheme is inspired to an original document held by Ratnoffs heirs (see [208]).
- 60.
e.g., Nuclear Magnetic Resonance (NMR) spectroscopy, X-ray crystallography.
- 61.
See [237] for historical evolution and future trends in MD simulations complexity with respect to computational power growth.
- 62.
This can easily be applied to non-Newtonian fluids, if necessary. However, for blood flows we usually assume the fluid phase being the blood plasma which is a Newtonian fluid under physiological conditions.
- 63.
approximated numerically in the simulations.
- 64.
See, e.g., [192] for platelet simulations.
- 65.
In a similar way as in Smoothed Particle Hydrodynamics (SPH) [146, 175] where the interpolation kernel is usually truncated to have a compact support. The SPH method differs significantly from many other particle methods because the equations of motion for the fictitious particles in SPH are derived directly from the partial differential equations of fluid mechanics by integration using an interpolation kernel [66].
- 66.
We use the same notation of particles position vectors as in the description of the DPD method.
- 67.
The net force acting on the particle i is \(\boldsymbol{f}_{i} =\sum _{i\neq j}\boldsymbol{f}_{ij}\).
- 68.
It uses similar principles as the Monte Carlo method in sub-microscale models.
- 69.
See [93] for the relation between this stochastic approach and continuous deterministic reaction rate equations.
- 70.
This equation is also known as Smoluchowski coagulation equation [221].
- 71.
The size can be, e.g., particle volume, mass, or dimension.
- 72.
Also called breakage function.
- 73.
A basis for this approach comes from atmospheric science where it was used to describe cloud formation [28].
- 74.
See p. 555 for details.
- 75.
The subscript Ia refers to the chemical notation for fibrin.
- 76.
See p. 554 for details.
- 77.
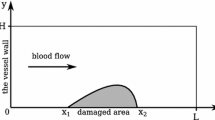
The dimensions are normalized using the vessel radius (half-diameter) R = 3. 1 mm.
- 78.
We should clarify that the simplification introduced assigning a pivotal role to prothrombinase and summarizing in one equation the complex process leading to its production makes sense only in the framework of the normal physiological process. If we have to consider any type of pathology referring, e.g., to a defective or missing factor, the model has to incorporate the equations involving the dynamics related to that specific factor. In other words, the model is conceived in an elastic way, adapting the number of equations to the complexity that needs to be taken into account.
- 79.
In contrast to a linear, continuous change in the previous model.
- 80.
I.e. a homogeneous Neumann condition in the context of this model.
- 81.
We don’t speak here about time-scales explicitly, however it is clear that each of the sub-models, depending on its resolved spatial scale, has an associated time scale that is able to treat (within a reasonable computational time and accuracy).
- 82.
In contrast with spatially two- or three-dimensional models.
- 83.
Square brackets are used to distinguish the concentrations of the corresponding chemicals from their names.
- 84.
released from an injured vessel wall (Tissue Factor TF)
- 85.
From the biochemical point of view the first term on the right-hand side of (7.73) has a dubious interpretation. Actually VIIa is present in small quantities but its production has to come from VII and is mediated by VIIa itself (positive loop), in addition to the Xa coming out at the initiation stage. All this is replaced by a constant stimulus α. Thus it should be kept in mind that this model is a shortcut. Actually, it bypasses the action of the tenase complex, which in turn involves VIIIa and IXa.
- 86.
This paper by H.C. Hemker et al. is entitled Is there value in kinetic modeling of thrombin generation? No (unless…). This review presents a summary of models showing the number of reactions they take into account, using the number of rate constants, based on the amount of cited papers. The rather critical point of view from this paper is balanced by another paper in the same issue of the journal written by K.G. Mann, under an almost identical title Is there value in kinetic modeling of thrombin generation? Yes [159].
- 87.
in one or more spatial dimensions
- 88.
For homogeneous models the Initial Value Problem is solved subject to initial data. By adding spatial variability to concentrations, the Initial Boundary Value Problem has to be solved using both, initial and boundary conditions.
- 89.
See [80] for details of derivation and use.
- 90.
See the paragraph on Euler–Lagrangian Particle Tracking methods (ELPT) in the section on microscale coagulation methods.
- 91.
Subject to appropriate initial and boundary conditions.
- 92.
Being positive, e.g., in the fluid (blood) and negative in the solid (thrombus).
- 93.
In Lagrangian particle tracking form.
- 94.
- 95.
Platelet does not get activated immediately, but only after a certain time t act.
- 96.
The thrombus model employs a composite structure with an impermeable core (activated platelets and fibrin) and a permeable shell (fibrin cap).
- 97.
The shear-rate is defined as \(\dot{\gamma }= 2\sqrt{\boldsymbol{D}: \boldsymbol{D}}\) where \(\boldsymbol{D} = (\nabla \boldsymbol{u} + \nabla \boldsymbol{u}^{T})/2\) is the symmetric part of velocity gradient.
- 98.
The upper-convected derivative \(\stackrel{\triangledown }{\boldsymbol{M}}\) of a tensor \(\boldsymbol{M}\) is defined using the classical material time-derivative \(\dot{\boldsymbol{M}}\) and the symmetric resp. skew-symmetric parts of the velocity gradient \(\boldsymbol{D}\) resp. \(\boldsymbol{W}\) as \(\stackrel{\triangledown }{\boldsymbol{M}} =\dot{\boldsymbol{ M}} -\boldsymbol{WM} + \boldsymbol{MW} - (\boldsymbol{DM} + \boldsymbol{MD})\).
- 99.
See [6] for the complete algorithm of platelet activation depending on the values of \(\mathcal{A}\).
- 100.
References
W.C. Aird, Vascular bed-specific thrombosis. J. Thromb. Haemost. 5(Suppl. 1), 283–291 (2007)
B.J. Alder, T.E. Wainwright, Phase transition for a hard sphere system. J. Chem. Phys. 27(5), 1208–1209 (1957)
D.J. Aldous, Deterministic and stochastic models for coalescence (aggregation and coagulation): a review of the mean-field theory for probabilists. Bernoulli 5(1), 3–48 (1999)
Y. Alemu, D. Bluestein, Flow-induced platelet activation and damage accumulation in a mechanical heart valve: numerical studies. Artif. Organs 31(9), 677–688 (2007)
A. Amadei, A.B.M. Linssen, H.J.C. Berendsen, Essential dynamics of proteins. Proteins Struct. Funct. Genet. 17(4), 412–425 (1993)
M. Anand, K.R. Rajagopal, A mathematical model to describe the change in the constitutive character of blood due to platelet activation. C. R. Mec. 330(8), 557–562 (2002)
M. Anand, K.R. Rajagopal, A shear-thinning viscoelastic fluid model for describing the flow of blood. Int. J. Cardiovasc. Med. Sci. 4(2), 59–68 (2004)
M. Anand, K. Rajagopal, K.R. Rajagopal, A model incorporating some of the mechanical and biochemical factors underlying clot formation and dissolution in flowing blood. J. Theor. Med. 5(3–4), 183–218 (2003)
M. Anand, K. Rajagopal, K.R. Rajagopal, A model for the formation and lysis of blood clots. Pathophysiol. Haemost. Thromb. 34(2–3), 109–120 (2005)
M. Anand, K. Rajagopal, K.R. Rajagopal, A model for the formation, growth, and lysis of clots in quiescent plasma. A comparison between the effects of antithrombin III deficiency and protein C deficiency. J. Theor. Biol. 253(4), 725–738 (2008)
M. Anand, J. Kwack, A. Masud, A new generalized Oldroid-b model for blood flow in complex geometries. Int. J. Eng. Sci. 72, 78–88 (2013)
S.T. Anning, The historical aspects of venous thrombosis. Med. Hist. 1(1), 28–37 (1957)
F.I. Ataullakhanov, G.T. Guria, V.I. Sarbash, R.I. Volkova, Spatiotemporal dynamics of clotting and pattern formation in human blood. Biochim. Biophys. Acta Gen. Subj. 1425(3), 453–468 (1998)
F. Bachmann, The discovery of factor X: a personal reminiscence. Thromb. Haemost. 98(1), 16–19 (2007)
P. Bagchi, Mesoscale simulation of blood flow in small vessels. Biophys. J. 92, 1858–1877 (2007)
C.N. Bagot, R. Arya, Virchow and his triad: a question of attribution. Br. J. Haematol. 143(2), 180–190 (2008)
I. Bahar, A.J. Rader, Coarse-grained normal mode analysis in structural biology. Curr. Opin. Struct. Biol. 15, 586–592 (2005)
F. Bai, Z. Wu, J. Jin, P. Hochendoner, J. Xing, Slow protein conformational change, Allostery and Network Dynamics, in Protein-Protein Interactions - Computational and Experimental Tools (InTech, Croatia, 2012), pp. 169–188
L. Baronciani, P.M. Manucci, The molecular basis of von Willebrand disease, in Molecular Hematology, chap. 19, 3rd edn., ed. by D. Provan, J.G. Gribben (Wiley-Blackwell, London, 2010), pp. 233–245
C. Basciano, C. Kleinstreuer, S. Hyun, E.A. Finol, A relation between near-wall particle-hemodynamics and onset of thrombus formation in abdominal aortic aneurysms. Ann. Biomed. Eng. 39(7), 2010–2026 (2011)
R.C. Becker, Cell-based models of coagulation: a paradigm in evolution. J. Thromb. Thrombolysis 20(1), 65–68 (2005)
E. Beltrami, J. Jesty, Mathematical analysis of activation thresholds in enzyme-catalyzed positive feedbacks: application to the feedbacks of blood coagulation. Proc. Natl. Acad. Sci. USA 92(19), 8744–8748 (1995)
T.K. Belval, J.D. Hellums, Analysis of shear-induced platelet aggregation with population balance mathematics. Biophys. J. 50(3), 479–487 (1986)
J. Bernard, J.P. Soulier, Sur une nouvelle variété de dystrophie thrombocytaire hémorragipare congénitale. Sem. Hôp. Paris 24, 3217–3223 (1948)
J. Bernsdorf, S.E. Harrison, S.M. Smith, P.V. Lawford, D.R. Hose, Concurrent numerical simulation of flow and blood clotting using the lattice Boltzmann technique. Int. J. Bioinform. Res. Appl. 2(4), 371–380 (2006)
J. Bernsdorf, S.E. Harrison, S.M. Smith, P.V. Lawford, D.R. Hose, Applying the lattice Boltzmann technique to biofluids: a novel approach to simulate blood coagulation. Comput. Math. Appl. 55(7), 1408–1414 (2008)
E.X. Berry, A mathematical framework for cloud models. J. Atmos. Sci. 26, 109–111 (1969)
J. Biasetti, P.G. Spazzini, J. Swedenborg, T. Christian Gasser, An integrated fluid-chemical model toward modeling the formation of intra-luminal thrombus in abdominal aortic aneurysms. Front. Physiol. 3, 1–16 (2012)
T. Bodnár, On the use of non-linear TVD filters in finite-volume simulations, in Algoritmy 2012 Proceedings of Contributed Papers and Posters, Bratislava. Faculty of Civil Engineering, Slovak University of Technology, pp. 190–199 (2012)
T. Bodnár, J. Příhoda, Numerical simulation of turbulent free-surface flow in curved channel. J. Flow Turbulence Combust. 76(4), 429–442 (2006)
T. Bodnár, A. Sequeira, Numerical simulation of the coagulation dynamics of blood. Comput. Math. Methods Med. 9(2), 83–104 (2008)
T. Bodnár, A. Sequeira, Numerical study of the significance of the non-Newtonian nature of blood in steady flow through a stenosed vessel, in Advances in Mathematical Fluid Mechanics, ed. by R. Rannacher, A. Sequeira (Springer, Berlin, 2010), pp. 83–104
T. Bodnár, K.R. Rajagopal, A. Sequeira, Simulation of the three-dimensional flow of blood using a shear-thinning viscoelastic fluid model. Math. Model. Nat. Phenom. 6(5), 1–24 (2011)
T. Bodnár, A. Sequeira, M. Prosi, On the shear-thinning and viscoelastic effects of blood flow under various flow rates. Appl. Math. Comput. 217(11), 5055–5067 (2011)
K. Boryczko, W. Dzwinela, D.A. Yuen, Modeling fibrin aggregation in blood flow with discrete-particles. Comput. Methods Programs Biomed. 75, 181–194 (2004)
K. Boryczko, D.A.Yuen, W. Dzwinel, Modeling mesoscopic fluids with discrete-particles – methods, algorithms, and results, in Finely Dispersed Particles: Micro-, Nano-, and Atto-Engineering (CRC Press, West Palm Beach, 2005), pp. 715–778
D.B. Brewer, Max Schultze (1865), G. Bizzozero (1882) and the discovery of the platelet. Br. J. Haematol. 133(3), 251–258 (2006)
L. Brugnano, F. Di Patti, G. Longo, An “incremental” mathematical model for Immune Thrombocytopenic Purpura (ITP). Math. Comput. Model. 42(11–12), 1299–1314 (2005)
K.E. Brummel-Ziedins, T. Orfeo, M. Gissel, K.G. Mann, F.R. Rosendaal, Factor Xa generation by computational modeling: an additional discriminator to thrombin generation evaluation. PLoS ONE 7(1), e29178 (2012)
K. Brummel-Ziedins, Models for thrombin generation and risk of disease. J. Thromb. Haemost. 11(Suppl.1), 212–223 (2013)
D. Brune, S. Kim, Predicting protein diffusion coefficients. Proc. Natl. Acad. Sci. USA 90(9), 3835–3839 (1993)
S. Butenas, T. Orfeo, M.T. Gissel, K.E. Brummel, K.G. Mann, The significance of circulating factor IXa in blood. J. Biol. Chem. 279(22), 22875–22882 (2004)
M.S. Chatterjee, W.S. Denney, H. Jing, S.L. Diamond, Systems biology of coagulation initiation: kinetics of thrombin generation in resting and activated human blood. PLoS Comput. Biol. 6(9), 1–24 (2010)
R. Chaturvedi, C. Huang, B. Kazmierczak, T. Schneider, J.A. Izaguirre, T. Glimm, H.G.E. Hentschel, J.A. Glazier, S.A. Newman, M.S. Alber, On multiscale approaches to three-dimensional modelling of morphogenesis. J. R. Soc. Interface 2(3), 237–253 (2005)
T. Cickovski, K. Aras, M.S. Alber, J.A. Izaguirre, M. Swat, J.A. Glazier, R.M.H. Merks, T. Glimm, H.G.E. Hentschel, S.A. Newman, From genes to organisms via the cell a problem-solving environment for multicellular development. Comput. Sci. Eng. 9(4), 50–60 (2007)
S. Cito, M.D. Mazzeo, L. Badimon, A review of macroscopic thrombus modeling methods. Thromb. Res. 131(2), 116–124 (2013)
K.J. Clementson, Platelets disorders, in Molecular Hematology, chap. 20, 3rd edn., ed. by D. Provan, J.G. Gribben (Wiley-Blackwell, London, 2010), pp. 246–258
B.S. Coller, A brief history of ideas about platelets in health and disease, in Platelets (Academic, New York, 2007), pp. xxiii–xlii
B. Cooper, Osler’s role in defining the third corpuscle, or “blood plates”. Proc. (Bayl. Univ. Med. Cent.) 18(4), 376–378 (2005)
J.M. Coutinho, J.M. Ferro, P. Canh ao, F. Barinagarrementeria, C. Cantú, M.-G. Bousser, J. Stam, Cerebral venous and sinus thrombosis in women. Stroke 40(7), 2356–2361 (2009)
K.J. Croce, M. Sakuma, D.I. Simon, Platelet-leukocyte-endothelial cross talk, in Platelets in Hematologic and Cardiovascular Disorders: A Clinical Handbook (Cambridge University Press, Cambridge, 2007), pp. 106–123
L.M. Crowl, A.L. Fogelson, Computational model of whole blood exhibiting lateral platelet motion induced by red blood cells. Int. J. Numer. Method Biomed. Eng. 26, 471–487 (2010)
S. Cunha Orfao, G. Jank, K. Mottaghy, S. Walcher, E. Zerz, Qualitative properties and stabilizability of a model for blood thrombin formation. J. Math. Anal. Appl. 346, 218–226 (2008)
B. Dahlbäck, Blood coagulation and its regulation by anticoagulant pathways: genetic pathogenesis of bleeding and thrombotic diseases. J. Inter. Med. 257(3), 209–223 (2005)
B. Dählbeck, A. Hillarp, Molecular coagulation and thrombophilia, in Molecular Hematology, chap. 17, 3rd edn., ed. by D. Provan, J.G. Gribben (Wiley-Blackwell, London, 2010), pp. 208–218
G. Davì, C. Patrono, Platelets activation and atherothrombosis. N. Engl. J. Med. 367, 2482–2494 (2007)
E.W. Davie, O.D. Ratnoff, Waterfall sequence for intrinsic blood clotting. Science 145(3638), 1310–1312 (1964)
S.L. Diamond, S. Anand, Inner clot diffusion and permeation during fibrinolysis. Biophys. J. 65(6), 2622–2643 (1993)
F. Dong, B. Olsen, N.A. Baker, Computational methods for biomolecular electrostatics, in Biophysical Tools for Biologists, Volume One: In Vitro Techniques, Volume 84 of Methods in Cell Biology (Elsevier, Amsterdam, 2008), pp. 843–870
R.L. Drake, A general mathematical survey of the coagulation equation, in Topics in Current Aerosol Research (Part 2), Volume 3 of International Reviews in Aerosol Physics and Chemistry, ed. by G.M. Hidy, J.R. Brock (Pergamon, Oxford, 1972), pp. 201–376
R.L. Drake, The scalar transport equation of coalescence theory: moments and kernels. J. Atmos. Sci. 29, 537–547 (1972)
P.B. Dubovskii, Mathematical theory of coagulation. Technical report, Seoul National University, Research Institute of Mathematics, Global Analysis Research Center (1994)
W. Dzwinel, K. Boryczko, D.A. Yuen, A discrete-particle model of blood dynamics in capillary vessels. J. Colloid Interface Sci. 258, 163–173 (2003)
W. Dzwinel, D.A. Yuen, K. Boryczko, Bridging diverse physical scales with the discrete-particle paradigm in modeling colloidal dynamics with mesoscopic features. Chem. Eng. Sci. 61, 2169–2185 (2006)
K.-E. Eilertsen, B. Østerud, The role of blood cells and their microparticles in blood coagulation. Biochem. Soc. Trans. 33(2), 418–422 (2005)
B. Engquist, P. Lötstedt, B. Sjögreen, Nonlinear filters for efficient shock computation. Math. Comput. 52(186), 509–537 (1989)
E.A. Ermakova, M.A. Panteleev, E.E. Shnol, Blood coagulation and propagation of autowaves in flow. Pathophysiol. Haemost. Thromb. 34(2–3), 135–142 (2006)
A. Farina, A. Fasano, J. Mizerski, A new model for blood flow in fenestrated capillaries with application to ultrafiltration in kidney glomeruli. A.M.S.A. (2014, to appear)
A. Fasano, R.F. Santos, A. Sequeira, Blood coagulation: a puzzle for biologists, a maze for mathematicians, in Modeling of Physiological Flows, Volume 5 of MS&A – Modeling, Simulation and Applications (Springer, Milan, 2012), pp. 41–75
A. Fasano, J. Pavlova, A. Sequeira, A synthetic model for blood coagulation including blood slip at the vessel wall. Clin. Hemorheol. Microcirc. 54(1), 1–14 (2013)
D.A. Fedosov, G.E. Karniadakis, Triple-decker: interfacing atomistic–mesoscopic–continuum flow regimes. J. Comput. Phys. 228(4), 1157–1171 (2009)
D.A. Fedosov, H. Noguchi, G. Gompper, Multiscale modeling of blood flow: from single cells to blood rheology. Biomech. Model. Mechanobiol. 1–20 (2013). doi:10.1007/s10237-013-0497-9
N. Filipovic, M. Kojic, A. Tsuda, Modelling thrombosis using dissipative particle dynamics method. Philos. Trans. R. Soc. A 366, 3265–3279 (2008)
M.H. Flamm, S.L. Diamond, Multiscale systems biology and physics of thrombosis under flow. Ann. Biomed. Eng. 40(11), 2355–2364 (2012)
A.L. Fogelson, R.D. Guy, Platelet-wall interactions in continuum models of platelet thrombosis: formulation and numerical solution. Math. Med. Biol. 21(4), 293–334 (2004)
A.L. Fogelson, R.D. Guy, Immersed-boundary-type models of intravascular platelet aggregation. Comput. Methods Appl. Mech. Eng. 197, 2087–2104 (2008)
A.L. Fogelson, J.P. Keener, Toward an understanding of fibrin branching structure. Phys. Rev. E 81(5), 051922-1–051922-9 (2010)
A.L. Fogelson, N. Tania, Coagulation under flow: the influence of flow-mediated transport on the initiation and inhibition of coagulation. Pathophysiol. Haemost. Thromb. 34(2–3), 91–108 (2006)
C. Forrey, M. Muthukumar, Langevin dynamics simulations of genome packing in bacteriophage. Biophys. J. 91, 25–41 (2006)
W.B. Foster, M.E. Nesheim, K.G. Mann, The factor Xa-catalyzed activation of factor V. J. Biol. Chem. 258(22), 13970–13977 (1983)
S.K. Friedlander, On the particle size spectrum of a condensing vapor. Phys. Fluids 3(5), 693–696 (1960)
S. Fuchigami, S. Omori, M. Ikeguchi, A. Kidera, Normal mode analysis of protein dynamics in a non-Eckart frame. J. Chem. Phys. 132, 104109-1–104109-7 (2010)
R.R. Gabdoulline, R.C. Wade, Brownian dynamics simulation of protein-protein diffusional encounter. Methods 14(3), 329–341 (1998)
D. Gailani, A. Zivelin, D. Sinha, P.N. Walsh, Do platelets synthesize factor XI? J. Thromb. Haemost. 2(10), 1709–1712 (2004)
R.B. Gayle, C.R. Maliszewski, S.D. Gimpel, M.A. Schoenborn, R.G. Caspary, C. Richards, K. Brasel, V. Price, J.H. Drosopoulos, N. Islam, T.N. Alyonycheva, M.J. Broekman, A.J. Marcus, Inhibition of platelet function by recombinant soluble ecto-ADPase/CD39. J. Clin. Investig. 101(9), 1851–1859 (1998)
V. Gazzaniga, L. Ottini, The discovery of platelets and their function. Vesalius VII(1), 22–26 (2001)
D.T. Gillespie, A general method for numerically simulating the stochastic time evolution of coupled chemical reactions. J. Comput. Phys. 22(4), 403–434 (1976)
D.T. Gillespie, Exact stochastic simulation of coupled chemical reactions. J. Phys. Chem. 81(25), 2340–2361 (1977)
D.T. Gillespie, Stochastic simulation of chemical kinetics. Annu. Rev. Phys. Chem. 58, 35–55 (2007)
D.T. Gillespie, Simulation methods in systems biology, in Formal Methods for Computational Systems Biology, Volume 5016 of Lecture Notes in Computer Science, ed. by M. Bernardo, P. Degano, G. Zavattaro (Springer, Berlin, 2008), pp. 125–167
D.T. Gillespie, Deterministic limit of stochastic chemical kinetics. J. Phys. Chem. B 113(6), 1640–1644 (2009)
W.E. Glanzmann, Hereditäre hämorrhägische Thrombasthenie. ein Beitrag zur Pathologie der Blutplättchen. Jahrb. Kinderheilkund. 88(1–42), 113–141 (1918)
J.A. Glazier, F. Graner, Simulation of the differential adhesion driven rearrangement of biological cells. Phys. Rev. E 47(3), 2128–2154 (1993)
G.H. Goldsmith, H. Saito, O.D. Ratnoff, The activation of plasminogen by Hageman factor (factor XII) and Hageman factor fragments. J. Clin. Investig. 62(1), 54–60 (1978)
M. Grigioni, C. Daniele, U. Morbiducci, G. D’Avenio, G. Di Benedetto, V. Barbaro, The power-law mathematical model for blood damage prediction: analytical developments and physical inconsistencies. Artif. Organs 28(5), 467–475 (2004)
M. Grigioni, U. Morbiducci, G. D’Avenio, G. Di Benedetto, C. Del Gaudio, A novel formulation for blood trauma prediction by a modified power-law mathematical model. Biomech. Model. Mechanobiol. 4(4), 249–260 (2005)
K.G. Guria, A.R. Gagarina, G.T. Guria, Instabilities in fibrinolytic regulatory system. theoretical analysis of blow-up phenomena. J. Theor. Biol. 304, 27–38 (2012)
R.I. Handin, Inherited platelet disorders. Am. Soc. Hematology 2005(1), 396–402 (2005)
S.E. Harrison, S.M. Smith, J. Bernsdorf, D.R. Hose, P.V. Lawford, Application and validation of the lattice Boltzmann method for modelling flow-related clotting. J. Biomech. 40(13), 3023–3028 (2007)
S. Hayward, B.L. de Groot, Normal modes and essential dynamics, in Molecular Modeling of Proteins, Volume 443 of Methods in Molecular Biology (Humana Press, Clifton, 2008), pp. 89–106
H.C. Hemker, S. Kerdelo, R.M.W. Kremers, Is there value in kinetic modeling of thrombin generation? No (unless…). J. Thromb. Haemost. 10(8), 1470–1477 (2012)
O. Hetland, A.B. Brovold, R. Holme, G. Gaudernack, H. Prydz, Thromboplastin (tissue factor) in plasma membranes of human monocytes. Biochem. J. 228(3), 735–743 (1985)
M.F. Hockin, K.C. Jones, S.J. Everse, K.G. Mann, A model for the stoichiometric regulation of blood coagulation. J. Biol. Chem. 277(21), 18322–18333 (2002)
M. Hoffman, Remodeling the blood coagulation cascade. J. Thromb. Thrombolysis 16(1–2), 17–20 (2003)
B. Honig, A. Nicholls, Classical electrostatics in biology and chemistry. Science 268(5214), 1144–1149 (1995)
P.J. Hoogerbrugge, J.M.V.A. Koelman, Simulating microscopic hydrodynamic phenomena with dissipative particle dynamics. Europhys. Lett. 19(3), 155–160 (1992)
W.H. Howell, E. Holt, Two new factors in blood coagulation: heparin and pro-antithrombin. Am. J. Physiol. 47, 228–241 (1918)
P.Y. Huang, J.D. Hellums, Aggregation and disaggregation kinetics of human blood platelets: part I. Development and validation of a population balance method. Biophys. J. 65(1), 334–343 (1993)
P.Y. Huang, J.D. Hellums, Aggregation and disaggregation kinetics of human blood platelets: part III. The disaggregation under shear stress of platelet aggregates. Biophys. J. 65(1), 354–361 (1993)
S.J. Hund, J.F. Antaki, M. Massoudi, On the representation of turbulent stresses for computing blood damage. Int. J. Eng. Sci. 48(11), 1325–1331 (2010)
S. Hyun, C. Kleinstreuer, J.P. Archie Jr., Computational particle-hemodynamics analysis and geometric reconstruction after carotid endarterectomy. Comput. Biol. Med. 31, 365–384 (2001)
Y. Imai, H. Kondo, T. Ishikawa, C.T. Lim, T. Yamaguchi, Modeling of hemodynamics arising from malaria infection. J. Biomech. 43, 1386–1393 (2010)
G.I. Ingram, The history of hemophilia. J. Clin. Pathol. 29(6), 469–479 (1976)
B. Isralewitz, J. Baudry, J. Gullingsrud, D. Kosztin, K. Schulten, Steered molecular dynamics investigations of protein function. J. Mol. Graph. Model. 19, 13–25 (2001)
A. Jameson, Time dependent calculations using multigrid, with applications to unsteady flows past airfoils and wings, in AIAA 10th Computational Fluid Dynamics Conference, Honolulu, Paper 91-1596, June 1991
A. Jameson, W. Schmidt, E. Turkel, Numerical solutions of the Euler equations by finite volume methods using Runge-Kutta time-stepping Schemes, in AIAA 14th Fluid and Plasma Dynamic Conference, Palo Alto, Paper 81-1259, June 1981
I. Johansson, N Lynöe, Medicine & Philosophy: A Twenty-First Century Introduction (Ontos Verlag, Frankfurt, 2008)
K.C. Jones, K.G. Mann, A model for the tissue factor pathway to thrombin. II. A mathematical simulation. J. Biol. Chem. 269(37), 23367–23373 (1994)
H. Kamada, K.I. Tsubota, M. Nakamura, S. Wada, T. Ishikawa, T. Yamaguchi, A three-dimensional particle simulation of the formation and collapse of a primary thrombus. Int. J. Numer. Methods Biomed. Eng. 26, 488–500 (2010)
H. Kamada, Y. Imai, M. Nakamura, T. Ishikawa, T. Yamaguchi, Computational analysis on the mechanical interaction between a thrombus and red blood cells: possible causes of membrane damage of red blood cells at microvessels. Med. Eng. Phys. 34, 1411–1420 (2012)
H. Kamada, Y. Imai, M. Nakamura, T. Ishikawa, T. Yamaguchi, Computational study on thrombus formation regulated by platelet glycoprotein and blood flow shear. Microvasc. Res. 89, 95–106 (2013)
M. Karplus, J. Kuriyan, Molecular dynamics and protein function. Proc. Natl. Acad. Sci. USA 102(19), 6679–6685 (2005)
J. Keener, J. Sneyd, Muscle, in Mathematical Physiology, Volume 8 of Interdisciplinary Applied Mathematics, chap. 18 (Springer, New York, 1998), pp. 542–578
J.G. Kelton, T.E. Warkentin, Heparin-induced thrombocytopenia: a historical perspective. Blood 112(7), 2607–2616 (2008)
M.A. Khanin, V.V. Semenov, A mathematical model of the kinetics of blood coagulation. J. Theor. Biol. 136(2), 127–134 (1989)
O.V. Kim, Z. Xu, E.D. Rosen, M.S. Alber, Fibrin networks regulate protein transport during thrombus development. PLoS Comput. Biol. 9(6), e1003095 (2013)
P. Kleinbongard, R. Schulz, T. Rassaf, T. Lauer, A. Dejam, T. Jax, I. Kumara, P. Gharini, S. Kabanova, B. Ozüyaman, H.G. Schnürch, A. Gödecke, A.A. Weber, M. Robenek, H. Robenek, W. Bloch, P. Rösen, M. Kelm, Red blood cells express a functional endothelial nitric oxide synthase. Blood 107(7), 2943–2951 (2006)
C. Kleinstreuer, J.R. Buchanan, M. Lei, G. A. Truskey, Computational analysis of particle hemodynamics and prediction of the onset of arterial diseases, in Biomechanical Systems, Techniques and Applications, Volume II. Cardiovascular Techniques (CRC Press, West Palm Beach, 2001)
A.E. Kogan, D.V. Kardakov, M.A. Khanin, Analysis of the activated partial thromboplastin time test using mathematical modeling. Thromb. Res. 101(4), 299–310 (2001)
H. Kondo, Y. Imai, T. Ishikawa, K.-I. Tsubota, T. Yamaguchi, Hemodynamic analysis of microcirculation in malaria infection. Ann. Biomed. Eng. 37(4), 702–709 (2009)
S. Koshizuka, Y. Oka, Moving-particle semi-implicit method for fragmentation of incompressible fluid. Nucl. Sci. Eng. 123(3), 421–434 (1996)
M. Kostoglou, Extended cell average technique for the solution of coagulation equation. J. Colloid Interface Sci. 306(1), 72–81 (2007)
A.L. Kuharsky, A.L. Fogelson, Surface-mediated control of blood coagulation: the role of binding site densities and platelet deposition. Biophys. J. 80(3), 1050–1074 (2001)
K. Laki, L. Lóránd, On the solubility of fibrin clots. Science 108(2802), 280 (1948)
N. Lannoy, C. Hermans, The ‘royal disease’- haemophilia A or B? A haematological mystery is finally solved. Haemophilia 16(6), 843–847 (2010)
I.J. Laurenzi, S.L. Diamond, Monte Carlo simulation of the heterotypic aggregation kinetics of platelets and neutrophils. Biophys. J. 77(3), 1733–1746 (1999)
E.H. Lee, J. Hsin, M. Sotomayor, G. Comellas, K. Schulten, Discovery through the computational microscope. Structure 17, 1295–1306 (2009)
R.J. Leipold, T.A. Bozarth, A.L. Racanelli, I.B. Dicker, Mathematical model of serine protease inhibition in the tissue factor pathway to thrombin. J. Biol. Chem. 270(43), 25383–25387 (1995)
A. Leuprecht, K. Perktold, Computer simulation of non-Newtonian effects on blood flow in large arteries. Comput. Methods Biomech. Biomed. Eng. 4(2), 149–163 (2001)
S.N. Levine, Enzyme amplifier kinetics. Science 152(3722), 651–653 (1966)
M. Levitt, C. Sander, P.S. Stern, Protein normal-mode dynamics: trypsin inhibitor, crambin, ribonuclease and lysozyme. J. Mol. Biol. 181, 423–447 (1985)
L.D. Libersky, A.G. Petschek, T.C. Carney, J.R. Hipp, High strain lagrangian hydrodynamics: a three-dimensional SPH code for dynamic material response. J. Comput. Phys. 109(1), 67–75 (1993)
B.B.C. Lim, E.H. Lee, M. Sotomayor, K. Schulten, Molecular basis of fibrin clot elasticity. Structure 16, 449–459 (2008)
Y. Liu, W.K. Liu, Rheology of red blood cell aggregation by computer simulation. J. Comput. Phys. 220, 135–154 (2006)
Y. Liu, L. Zhang, X. Wang, W.K. Liu, Coupling of Navier-Stokes equations with protein molecular dynamics and its application to hemodynamics. Int. J. Numer. Methods Fluids 46(12), 1237–1252 (2004)
W.K. Liu, Y. Liu, D. Farrell, L. Zhang, X.S. Wang, Y. Fukui, N. Patankar, Y. Zhang, C. Bajaj, J. Lee, J. Hong, X. Chen, H. Hsu, Immersed finite element method and its applications to biological systems. Comput. Methods Appl. Mech. Eng. 195, 1722–1749 (2006)
K. Lo, W.S. Denney, S.L. Diamond, Stochastic modeling of blood coagulation initiation. Pathophysiol. Haemost. Thromb. 34(2–3), 80–90 (2006)
A.I. Lobanov, T.K. Starozhilova, The effect of convective flows on blood coagulation processes. Pathophysiol. Haemost. Thromb. 34(2–3), 121–134 (2006)
A.I. Lobanov, A.V. Nikolaev, T.K. Starozhilova, Mathematical model of fibrin polymerization. Math. Model. Nat. Phenom. 6(7), 55–69 (2011)
P.W. Longest, C. Kleinstreuer, Comparison of blood particle deposition models for non-parallel flow domains. J. Biomech. 36, 421–430 (2003)
P.W. Longest, C. Kleinstreuer, J.R. Buchanan, Efficient computation of micro-particle dynamics including wall effects. Comput. Fluids 33, 577–601 (2004)
J. Ma, Usefulness and limitations of normal mode analysis in modeling dynamics of biomolecular complexes. Structure 13, 373–380 (2005)
Y. Ma, J. Wang, S. Liang, C. Dong, Q. Du, Application of population dynamics to study heterotypic cell aggregations in the near-wall region of a shear flow. Cell. Mol. Bioeng. 3(1), 3–19 (2010)
R.G. MacFarlane, An enzyme cascade in the blood clotting mechanism, and its function as a biochemical amplifier. Nature 202(4931), 498–499 (1964)
K.G. Mann, Is there value in kinetic modeling of thrombin generation? Yes. J. Thromb. Haemost. 10(8), 1463–1469 (2012)
K.G. Mann, S. Butenas, K. Brummel, The dynamics of thrombin formation. Arterioscler. Thromb. Vasc. Biol. 23(1), 17–25 (2003)
K.G. Mann, K. Brummel-Ziedins, T. Orfeo, S. Butenas, Models of blood coagulation. Blood Cells Mol. Dis. 36(2), 108–117 (2006)
J.A. Marcum, The origin of the dispute over the discovery of heparin. J. Hist. Med. Allied Sci. 55(1), 37–66 (2000)
F. Martorana, A. Moro, On the kinetics of enzyme amplifier systems with negative feedback. Math. Biosci. 21(1–2), 77–84 (1974)
D. Massai, G. Soloperto, D. Gallo, X.Y. Xu, U. Morbiducci, Shear-induced platelet activation and its relationship with blood flow topology in a numerical model of stenosed carotid bifurcation. Eur. J. Mech. B Fluids 35, 92–101 (2012)
M.R. Maxey, B.K. Patel, Localized force representations for particles sedimenting in Stokes flow. Int. J. Multiphase Flow 27(9), 1603–1626 (2001)
J.A. McCammon, B.R. Gelin, M. Karplus, Dynamics of folded proteins. Nature 267(5612), 585–590 (1977)
J. McLean, The discovery of heparin. Circulation 19(1), 75–78 (1959)
S. Melchionna, A model for red blood cells in simulations of large-scale blood flows. Macromol. Theory Simul. 20(7), 548–561 (2011)
Z.A. Melzak, A scalar transport equation. Trans. Am. Math. Soc. 85(2), 547–560 (1957)
Z.A. Melzak, A scalar transport equation ii. Mich. Math. J. 4(3), 193–206 (1957)
A.D. Michelson, Platelets, 2nd edn. (Academic, New York, 2007)
G. Moiseyev, P.Z. Bar-Yoseph, No need for particle tracing: from accumulating fluid properties to novel blood coagulation model in the lattice Boltzmann method. J. Biomech. 43(5), 864–870 (2010)
G. Moiseyev, P.Z. Bar-Yoseph, Computational modeling of thrombosis as a tool in the design and optimization of vascular implants. J. Biomech. 46, 248–252 (2013)
G. Moiseyev, S. Givli, P.Z. Bar-Yoseph, Fibrin polymerization in blood coagulation-a statistical model. J. Biomech. 46(1), 26–30 (2013)
J.J. Monaghan, Smoothed particle hydrodynamics. Annu. Rev. Astron. Astrophys. 30(1), 543–574 (1992)
D.D. Monkovic, P.B. Tracy, Activation of human factor V by factor Xa and thrombin. Biochemistry 29(5), 1118–1128 (1990)
U. Morbiducci, R. Ponzini, M. Nobili, D. Massai, F.M. Montevecchi, D. Bluestein, A. Redaelli, Blood damage safety of prosthetic heart valves. Shear-induced platelet activation and local flow dynamics: a fluid–structure interaction approach. J. Biomech. 42, 1952–1960 (2009)
D. Mori, K. Yano, K.-i. Tsubota, T. Ishikawa, S. Wada, T. Yamaguchi, Computational study on effect of red blood cells on primary thrombus formation. Thromb. Res. 123, 114–121 (2008)
A. Moro, A.T. Bharucha-Reid. On the kinetics of enzyme amplifier systems. Math. Biosci. 5(3–4), 391–402 (1969)
L. Mountrakis, E. Lorenz, A.G. Hoekstra, Where do the platelets go? A simulation study of fully resolved blood flow through aneurysmal vessels. Interface Focus 3(2), 20120089 (2013)
M.E. Nesheim, W.M. Canfield, W. Kisiel, K.G. Mann, Studies of the capacity of factor Xa to protect factor Va from inactivation by activated protein C. J. Biol. Chem. 257(3), 1443–1447 (1982)
K.C. Ng, Y.L. Ng, M.Z. Yusoff, Development of a Lagrangian meshless flow solver based on the moving particle semi-implicit (MPS) method, in 4th International Conference on Energy and Environment 2013 (ICEE2013), Volume 16 of IOP Conference Series: Earth and Environmental Science, p. 012151 (2013)
M. Nobili, J. Sheriff, U. Morbiducci, A. Redaelli, D. Bluestein, Platelet activation due to hemodynamic shear stresses: damage accumulation model and comparison to in vitro measurements. ASAIO J. 54(1), 64–72 (2008)
P. Espa nol, Fluid particle model. Phys. Rev. E 57(3), 2930–2948 (1998)
Th. Orfeo, S. Butenas, K.E. Brummel-Ziedins, K.G. Mann, The tissue factor requirement in blood coagulation. J. Biol. Chem. 280, 42887–42896 (2005)
T. Orfeo, M. Gissel, S. Butenas, A. Undas, K.E. Brummel-Ziedins, K.G. Mann, Anticoagulants and the propagation phase of thrombin generation. PLoS ONE 6(11), e27852 (2011)
R. Ouared, B. Chopard, B. Stahl, D.A. Rüfenacht, H. Yilmaz, G. Courbebaisse, Thrombosis modeling in intracranial aneurysms: a lattice Boltzmann numerical algorithm. Comput. Phys. Commun. 179(1–3), 128–131 (2008)
P. Owren, Parahaemophilia. Haemorrhagic diathesis due to absence of a previously unknown clotting factor. Lancet 249(6449), 446–448 (1947)
M.A. Panteleev, M.V. Ovanesov, D.A. Kireev, A.M. Shibeko, E.I. Sinauridze, N.M. Ananyeva, A.A. Butylin, E.L. Saenko, F.I. Ataullakhanov, Spatial propagation and localization of blood coagulation are regulated by intrinsic and protein c pathways, respectively. Biophys. J. 90(5), 1489–1500 (2006)
V. Pappu, P. Bagchi, 3D computational modeling and simulation of leukocyte rolling adhesion and deformation. Comput. Biol. Med. 38, 738–753 (2008)
V. Pappu, S.K. Doddi, P. Bagchi, A computational study of leukocyte adhesion and its effect on flow pattern in microvessels. J. Theor. Biol. 254, 483–498 (2008)
I.V. Pivkin, P.D. Richardson, G. Karniadakis, Blood flow velocity effects and role of activation delay time on growth and form of platelet thrombi. Proc. Natl. Acad. Sci. USA 103(46), 17164–17169 (2006)
I.V. Pivkin, P.D. Richardson, G.E. Karniadakis, Effect of red blood cells on platelet aggregation. IEEE Eng. Med. Biol. Mag. 28(2), 32–37 (2009)
A. Podmore, M. Smith, G. Savidge, A. Alhaq, Real-time quantitative PCR analysis of factor XI mRNA variants in human platelets. J. Thromb. Haemost. 2(10), 1713–1719 (2004)
A.V. Pokhilko, F.I. Ataullakhanov, Contact activation of blood coagulation: trigger properties and hysteresis. J. Theor. Biol. 191(2), 213–219 (1998)
D. Raabe, Overview of the lattice Boltzmann method for nano- and microscale fluid dynamics in materials science and engineering. Model. Simul. Mater. Sci. Eng. 12(6), R13–R46 (2004)
A. Rahman, Correlations in the motion of atoms in liquid argon. Phys. Rev. 136(2A), A405–A411 (1964)
K.R. Rajagopal, A.R. Srinivasa, A thermodynamic frame work for rate type fluid models. J. Non-Newtonian Fluid Mech. 88(3), 207–227 (2000)
J.M. Ramstack, L. Zuckerman, L.F. Mockros, Shear-induced activation of platelets. J. Biomech. 12(2), 113–125 (1979)
O.D. Ratnoff, J.M. Rosenblum, Role of Hageman factor in the initiation of clotting by glass. Evidence that glass frees Hageman factor from inhibition. Am. J. Med. 25(2), 160–168 (1958)
R.L. Reddick, T.R. Griggs, M.A. Lamb, K.M. Brinkhous, Platelet adhesion to damaged coronary arteries: comparison in normal and von Willebrand disease swine. Proc. Natl. Acad. Sci. USA 79(16 I), 5076–5079 (1982)
D. Ribatti, E. Crivellato, Giulio Bizzozero and the discovery of platelets. Leuk. Res. 31(10), 1339–1341 (2007)
E. Richardson, Applications of a theoretical model for haemolysis in shear flow. Biorheology 12(1), 27–37 (1975)
F.R. Rickles, S. Patierno, P.M. Fernandez, Tissue factor, thrombin, and cancer. CHEST 124, 58S–68S (2003)
J.P. Riddel Jr., B.E. Aouizerat, C. Miaskowski, D.P. Lillicrap, Theories of blood coagulation. J. Pediatr. Oncol. Nurs. 24(3), 123–131 (2007)
J. Rivera, M.L. Lozano, L. Navarro-Núñez, V. Vicente, Platelet receptors and signaling in the dynamics of thrombus formation. Haematologica 94(5), 700–711 (2009)
H.R. Roberts, Oscar Ratnoff: his contributions to the golden era of coagulation research. Br. J. Haematol. 122(2), 180–192 (2003)
Z.M. Ruggeri, Perspectives series: cell adhesion in vascular biology – von Willebrand factor. J. Clin. Investig. 99(4), 559–564 (1997)
J.E. Sadler, Biochemistry and genetics of von Willebrand factor. Annu. Rev. Biochem. 67, 395–424 (1998)
B. Savage, J.J. Sixma, Z.M. Ruggeri, Functional self-association of von Willebrand factor during platelet adhesion under flow. Proc. Natl. Acad. Sci. USA 99(1), 425–430 (2002)
A.H. Schmaier, G. LaRusch, Factor XII: new life for an old protein. Thromb. Haemost. 104(5), 915–918 (2010)
A. Sequeira, R.F. Santos, T. Bodnár. Blood coagulation dynamics: mathematical modeling and stability results. Math. Biosci. Eng. 8(2), 425–443 (2011)
S.C. Shadden, S. Hendabadi, Potential fluid mechanic pathways of platelet activation. Biomech. Model. Mechanobiol. 12, 467–474 (2012)
V. Shankar, G.B. Wright, A.L. Fogelson, R.M. Kirbya, A study of different modeling choices for simulating platelets within the immersed boundary method. Appl. Numer. Math. 63, 58–77 (2013)
A.M. Shibeko, E.S. Lobanova, M.A. Panteleev, F.I. Ataullakhanov, Blood flow controls coagulation onset via the positive feedback of factor VII activation by factor Xa. BMC Syst. Biol. 4 (2010)
W. Shyy, M.-H. Chen, R. Mittal, H.S. Udaykumar, On the suppression of numerical oscillations using a non-linear filter. J. Comput. Phys. 102, 49–62 (1992)
L. Skjaerven, S.M. Hollup, N. Reuter, Normal mode analysis for proteins. J. Mol. Struct. THEOCHEM 898, 42–48 (2009)
S.A. Smith, The cell-based model of coagulation: State-of-the-Art Review. J. Vet. Emerg. Crit. Care. 19(1), 3–10 (2009)
D.J. Smith, M.J. Hounslow, W.R. Paterson, Aggregation and gelation - I. Analytical solutions for CST and batch operation. Chem. Eng. Sci. 49(7), 1025–1035 (1994)
M. V. Smoluchowski, Drei Vorträge über Diffusion, Brownsche Bewegung und Koagulation von Kolloidteilchen. Z. Phys. 17, 557–585 (1916)
J.S. Soares, J. Sheriff, D. Bluestein, A novel mathematical model of activation and sensitization of platelets subjected to dynamic stress histories. Biomech. Model. Mechanobiol. 12, 1127–1141 (2013)
X. Song, A.L. Throckmorton, H.G. Wood, J.F. Antaki, D.B. Olsen, Computational fluid dynamics prediction of blood damage in a centrifugal pump. Artif. Organs 27(10), 938–941 (2003)
E.N. Sorensen, G.W. Burgreen, W.R. Wagner, J.F. Antaki, Computational simulation of platelet deposition and activation: I. Model development and properties. Ann. Biomed. Eng. 27(4), 436–448 (1999)
E.N. Sorensen, G.W. Burgreen, W.R. Wagner, J.F. Antaki, Computational simulation of platelet deposition and activation: II, results for Poiseuille flow over collagen. Ann. Biomed. Eng. 27(4), 449–458 (1999)
R.F. Stevens, The history of haemophilia in the royal families of europe. Br. J. Haematol. 105(1), 25–32 (1999)
H. Stormorken, Paul A. Owren and the Golden Era of Haemostasis (Gazettebok, Sandvika, 2001)
O. Taboureau, O.H. Olsen, Computational study of coagulation factor VIIa’s affinity for phospholipid membranes. Eur. Biophys. J. 36, 133–144 (2007)
A.A. Tokarev, Yu.V. Krasotkina, M.V. Ovanesov, M.A. Panteleev, M.A. Azhigirova, V.A. Volpert, F.I. Ataullakhanov, A.A. Butilin, Spatial dynamics of contact-activated fibrin clot formation in vitro and in silico in haemophilia b: effects of severity and ahemphil b treatment. Math. Model. Nat. Phenom. 1(2), 124–137 (2006)
A. Tokarev, I. Sirakov, G. Panasenko, V. Volpert, E. Shnol, A. Butylin, F. Ataullakhanov, Continuous mathematical model of platelet thrombus formation in blood flow. Russ. J. Numer. Anal. Math. Model. 27(2), 191–212 (2012)
A. Tosenberger, V. Salnikov, N. Bessonov, E. Babushkina, V. Volpert, Particle dynamics methods of blood flow simulations. Math. Model. Nat. Phenom. 6(5), 320–332 (2011)
A. Tosenberger, F. Ataullakhanov, N. Bessonov, M. Panteleev, A. Tokarev, V. Volpert, Modelling of thrombus growth in flow with a DPD-PDE method. J. Theor. Biol. 337, 30–41 (2013)
S.D. Treadwell, B. Thanvi T.G. Robinson, Stroke in pregnancy and the puerperium. Postgrad. Med. J. 84(991), 238–245 (2008)
A. Trousseau, Phlegmatia alba dolens, in Clinique médicale de l’Hôtel-Dieu de Paris, vol. 3 (J.B. Balliére et Fils, Paris, 1865), pp. 654–712
D.M.F. Van Aalten, B.L. De Groot, J.B.C. Findlay, H.J.C. Berendsen, A. Amadei, A comparison of techniques for calculating protein essential dynamics. J. Comput. Chem. 18(2), 169–181 (1997)
W.F. Van Gunsteren, D. Bakowies, R. Baron, I. Chandrasekhar, M. Christen, X. Daura, P. Gee, D.P. Geerke, A. Glättli, P.H. Hünenberger, M.A. Kastenholz, C. Oostenbrink, M. Schenk, D. Trzesniak, N.F.A. Van Der Vegt, H.B. Yu, Biomolecular modeling: goals, problems, perspectives. Angew. Chem. Int. Ed. 45(25), 4064–4092 (2006)
B.O. Villoutreix, Structural bioinformatics: methods, concepts and applications to blood coagulation proteins. Curr. Protein Pept. Sci. 3, 341–364 (2002)
B.O. Villoutrei, O. Sperandio, In silico studies of blood coagulation proteins: from mosaic proteases to nonenzymatic cofactor inhibitors. Curr. Opin. Struct. Biol. 20, 168–179 (2010)
R.L.K. Virchow, Thrombose und Embolie. Gefässentzündung und septische Infektion, in Gesammelte Abhandlungen zur wissenschaftlichen Medicin (Von Meidinger & Sohn., Frankfurt am Main, 1856), pp. 219–732
G.J. Wagner, W.K. Liu, Coupling of atomistic and continuum simulations using a bridging scale decomposition. J. Comput. Phys. 190, 249–274 (2003)
C. Wagner, P. Steffen, S. Svetina, Aggregation of red blood cells: from rouleaux to clot formation. C. R. Phys. 14(6), 459–469 (2013)
F.J. Walker, P.W. Sexton, C.T. Esmon, The inhibition of blood coagulation by activated protein C through the selective inactivation of activated factor V. Biochim. Biophys. Acta 571(2), 333–342 (1979)
A.H. Schmaier, L.D. Dahl, A.J. Rozemuller, R.A. Roos, S.L. Wagner, R. Chung, W.E. Van Nostrand, Protease nexin-2/amyloid beta protein precursor. A tight-binding inhibitor of coagulation factor IXa. J. Clin. Invest. 92(5), 2540–2545 (1993)
N.-H.L. Wang, K.H. Keller, Augmented transport of extracellular solutes in concentrated erythrocyte suspensions in Couette flow. J. Colloid Interface Sci. 103(1), 210–225 (1985)
W. Wang, M.R. King, Multiscale modeling of platelet adhesion and thrombus growth. Ann. Biomed. Eng. 40(11), 2345–2354 (2012)
H. Wang, C. Junghans, K. Kremer, Comparative atomistic and coarse-grained study of water: what do we lose by coarse-graining? Eur. Phys. J. E 28, 221–229 (2009)
D. Wardrop, D. Keeling, The story of the discovery of heparin and warfarin. Br. J. Haematol. 141(6), 757–763 (2008)
F.F. Weller, Modeling, analysis, and simulation of thrombosis and hemostasis. Ph.D. thesis, Ruprecht–Karls–Universität Heidelberg, 2008
F.F. Weller, Platelet deposition in non-parallel flow: influence of shear stress and changes in surface reactivity. J. Math. Biol. 57(3), 333–359 (2008)
F.F. Weller, A free boundary problem modeling thrombus growth: model development and numerical simulation using the level set method. J. Math. Biol. 61(6), 805–818 (2010)
N.K. Wenger, Clinical characteristics of coronary heart disease in women: emphasis on gender differences. Cardiovasc. Res. 53(3), 558–567 (2002)
G.M. Willems, T. Lindhout, W.T. Hermens, H.C. Hemker, Simulation model for thrombin generation in plasma. Haemostasis 21(4), 197–207 (1991)
D.M. Wootton, C.P. Markou, S.R. Hanson, D.N. Ku, A mechanistic model of acute platelet accumulation in thrombogenic stenoses. Ann. Biomed. Eng. 29(4), 321–329 (2001)
I.S. Wright, Nomenclature of blood clotting factors. Can. Med. Assoc. J. 80(8), 659–661 (1959)
I.S. Wright, Nomenclature of blood clotting factors. Can. Med. Assoc. J. 86, 373–374 (1962)
J. Wu, C.K. Aidun, A method for direct simulation of flexible fiber suspensions using lattice Boltzmann equation with external boundary force. Int. J. Multiphase Flow 36, 202–209 (2010)
J. Wu, C.K. Aidun, Simulating 3D deformable particle suspensions using lattice Boltzmann method with discrete external boundary force. Int. J. Numer. Methods Fluids 62(7), 765–783 (2010)
J. Wu, B.M. Yun, A.M. Fallon, S.R. Hanson, C.K. Aidun, A.P Yoganathan, Numerical investigation of the effects of channel geometry on Platelet activation and blood damage. Ann. Biomed. Eng. 39(2), 897–910 (2011)
C.Q. Xu, Y.J. Zeng, H. Gregersen, Dynamic model of the role of platelets in the blood coagulation system. Med. Eng. Phys. 24(9), 587–593 (2002)
C. Xu, X.H. Xu, Y. Zeng, Y.W. Chen, Simulation of a mathematical model of the role of the TFPI in the extrinsic pathway of coagulation. Comput. Biol. Med. 35(5), 435–445 (2005)
Z. Xu, N. Chen, M.M. Kamocka, E.D. Rosen, M. Alber, A multiscale model of thrombus development. J. R. Soc. Interface 5, 705–722 (2008)
Z. Xu, N. Chen, S.C. Shadden, J.E. Marsden, M.M. Kamocka, E.D. Rosen, M. Alber, Study of blood flow impact on growth of thrombi using a multiscale model. Soft Matter 5(4), 769–779 (2009)
Z. Xu, J. Lioi, M. Alber, J. Mu, X. Liu, D.Z. Chen, M.M. Kamocka, E.D. Rosen, Combined experimental and simulation study of blood clot formation, in TIC-STH’09: 2009 IEEE Toronto International Conference - Science and Technology for Humanity, pp. 357–362 (2009)
Z. Xu, J. Lioi, J. Mu, M.M. Kamocka, X. Liu, D.Z. Chen, E.D. Rosen, M. Alber, A multiscale model of venous thrombus formation with surface-mediated control of blood coagulation cascade. Biophys. J. 98, 1723–1732 (2010)
Z. Xu, M. Kamocka, M. Alber, E.D. Rosen, Computational approaches to studying thrombus development. Arterioscler. Thromb. Vasc. Biol. 31, 500–505 (2011)
Z. Xu, O. Kim, M. Kamocka, E.D. Rosen, M. Alber, Multiscale models of thrombogenesis. Wiley Interdiscip. Rev. Syst. Biol. Med. 4(3), 237–246 (2012)
D. Xu, E. Kaliviotis, A. Munjiza, E. Avital, C. Ji, J. Williams, Large scale simulation of red blood cell aggregation in shear flows. J. Biomech. 46, 1810–1817 (2013)
T. Yamaguchi, T. Ishikawa, Y. Imai, N. Matsuki, M. Xenos, Y. Deng, D. Bluestein, Particle-based methods for multiscale modeling of blood flow in the circulation and in devices: challenges and future directions. Ann. Biomed. Eng. 38(3), 1225–1235 (2010)
K. Yano, D. Mori, K. Tsubota, T. Ishikawa, S. Wada, T. Yamaguchi, Analysis of destruction process of the primary thrombus under the influence of the blood flow. J. Biomech. Sci. Eng. 2(1), 34–44 (2007)
K.K. Yeleswarapu, J.F. Antaki, M.V. Kameneva, K.R. Rajagopal, A mathematical model for shear-induced hemolysis. Artif. Organs 19(7), 576–582 (1995)
M. E. Young, P. A. Carroad, R. L. Bell, Estimation of diffusion coefficients of proteins. Biotechnol. Bioeng. 22(5), 947–955 (1980)
V.I. Zarnitsina, A.V. Pokhilko, F.I. Ataullakhanov, A mathematical model for the spatio-temporal dynamics of intrinsic pathway of blood coagulation. I. The model description. Thromb. Res. 84(4), 225–236 (1996)
V.I. Zarnitsina, A.V. Pokhilko, F.I. Ataullakhanov, A mathematical model for the spatio-temporal dynamics of intrinsic pathway of blood coagulation. ii. Results. Thromb. Res. 84(5), 333–344 (1996)
V.I. Zarnitsina, F.I. Ataullakhanov, A.I. Lobanov, O.L. Morozova, Dynamics of spatially nonuniform patterning in the model of blood coagulation. Chaos 11(1), 57–70 (2001)
L. Zhang, A. Gerstenberger, X. Wang, W.K. Liu, Immersed finite element method. Comput. Methods Appl. Mech. Eng. 193, 2051–2067 (2004)
J. Zhang, P.C. Johnson, A.S. Popel, An immersed boundary lattice Boltzmann approach to simulate deformable liquid capsules and its application to microscopic blood flows. Phys. Biol. 4, 285–295 (2007)
J. Zhang, P.C. Johnson, A.S. Popel, Red blood cell aggregation and dissociation in shear flows simulated by lattice Boltzmann method. J. Biomech. 41, 47–55 (2008)
R.F.A. Zwaal, P. Comfurius, E.M. Bevers, Surface exposure of phosphatidylserine in pathological cells. Cell. Mol. Life Sci. 62(9), 971–988 (2005)
A.L. Zydney, C.K. Colton, Augmented solute transport in the shear flow of a concentrated suspension. PCH. Physicochem. Hydrodyn. 10(1), 77–96 (1988)
Acknowledgements
The financial support for the present project was partly provided by the Czech Science Foundation under the Grant No.201/09/0917 and by the Portuguese Science Foundation under The Research Center CEMAT-IST and under the Project EXCL/MAT-NAN/0114/2012.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Basel
About this chapter
Cite this chapter
Bodnár, T., Fasano, A., Sequeira, A. (2014). Mathematical Models for Blood Coagulation. In: Bodnár, T., Galdi, G., Nečasová, Š. (eds) Fluid-Structure Interaction and Biomedical Applications. Advances in Mathematical Fluid Mechanics. Birkhäuser, Basel. https://doi.org/10.1007/978-3-0348-0822-4_7
Download citation
DOI: https://doi.org/10.1007/978-3-0348-0822-4_7
Published:
Publisher Name: Birkhäuser, Basel
Print ISBN: 978-3-0348-0821-7
Online ISBN: 978-3-0348-0822-4
eBook Packages: Mathematics and StatisticsMathematics and Statistics (R0)