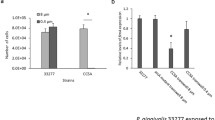
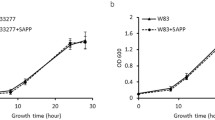
Abstract
Colonization and virulence of Porphyromonas gingivalis are enhanced through interactions with synergistic organisms in plaque biofilm communities. Conversely, some oral community inhabitants are antagonistic toward P. gingivalis. As many of the effectors of synergism and antagonism are proteinaceous, peptide-based inhibition of synergism or stimulation of antagonism are attractive strategies to reduce P. gingivalis pathogenicity and to prevent and treat periodontitis. Recent years have witnessed the development of peptides, and their functional mimics, designed to impede the assembly of adhesins, block the attachment of P. gingivalis to streptococcal substrata, or suppress the transcription and production of virulence factors. Coupled with advances in nanoparticle delivery systems, these approaches show promise for preventing the transition of the plaque biofilm from a commensal to a pathogenic entity.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Lamont RJ, Jenkinson HF. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev. 1998;62(4):1244–63.
Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol. 2018;16(12):745–59.
Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15(1):30–44.
Hajishengallis G, Lamont RJ. Breaking bad: manipulation of the host response by Porphyromonas gingivalis. Eur J Immunol. 2014;44(2):328–38.
Lamont RJ, Hajishengallis G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol Med. 2015;21(3):172–83.
Xie H. Biogenesis and function of Porphyromonas gingivalis outer membrane vesicles. Future Microbiol. 2015;10(9):1517–27.
Ebersole JL, Dawson D 3rd, Emecen-Huja P, Nagarajan R, Howard K, Grady ME, et al. The periodontal war: microbes and immunity. Periodontol 2000. 2017;75(1):52–115.
Smalley JW, Olczak T. Haem acquisition mechanisms of Porphyromonas gingivalis—strategies used in a polymicrobial community in a haem-limited host environment. Mol Oral Microbiol. 2017;32(1):1–23.
Nakayama K. Porphyromonas gingivalis and related bacteria: from colonial pigmentation to the type IX secretion system and gliding motility. J Periodontal Res. 2015;50(1):1–8.
Hajishengallis G, Lamont RJ. Dancing with the stars: how choreographed bacterial interactions dictate nososymbiocity and give rise to keystone pathogens, accessory pathogens, and pathobionts. Trends Microbiol. 2016;24:477–89.
Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nat Rev Microbiol. 2012;10(10):717–25.
Slots J, Gibbons RJ. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticus to oral surfaces and its possible role in colonization of the mouth and of periodontal pockets. Infect Immun. 1978;19(1):254–64.
Socransky SS, Haffajee AD, Ximenez-Fyvie LA, Feres M, Mager D. Ecological considerations in the treatment of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis periodontal infections. Periodontol 2000. 1999;20:341–62.
Tanaka S, Minami M, Murakami Y, Ogiwara T, Seto K, Shoji M, et al. The detection of Porphyromonas gingivalis, Prevotella intermedia and Actinobacillus actinomycetemcomitans in tooth, tongue and buccal mucosa plaques in children, using immunoslot blot assay (IBA). J Clin Pediatr Dent. 2006;30(3):251–6.
Tanaka S, Murakami Y, Seto K, Takamori K, Yosida M, Ochiai K, et al. The detection of Porphyromonas gingivalis, Prevotella intermedia, and Actinobacillus actinomycetemcomitans in the supragingival plaque of children with and without caries. Pediatr Dent. 2003;25(2):143–8.
Mayanagi G, Sato T, Shimauchi H, Takahashi N. Detection frequency of periodontitis-associated bacteria by polymerase chain reaction in subgingival and supragingival plaque of periodontitis and healthy subjects. Oral Microbiol Immunol. 2004;19(6):379–85.
Okada M, Hayashi F, Nagasaka N. Detection of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in dental plaque samples from children 2 to 12 years of age. J Clin Periodontol. 2000;27(10):763–8.
Ximenez-Fyvie LA, Haffajee AD, Socransky SS. Microbial composition of supra- and subgingival plaque in subjects with adult periodontitis. J Clin Periodontol. 2000;27(10):722–32.
Ximenez-Fyvie LA, Haffajee AD, Socransky SS. Comparison of the microbiota of supra- and subgingival plaque in health and periodontitis. J Clin Periodontol. 2000;27(9):648–57.
Van Winkelhoff AJ, Boutaga K. Transmission of periodontal bacteria and models of infection. J Clin Periodontol. 2005;32(Suppl 6):16–27.
Tanner AC, Milgrom PM, Kent R Jr, Mokeem SA, Page RC, Riedy CA, et al. The microbiota of young children from tooth and tongue samples. J Dent Res. 2002;81(1):53–7.
Yang EY, Tanner AC, Milgrom P, Mokeem SA, Riedy CA, Spadafora AT, et al. Periodontal pathogen detection in gingiva/tooth and tongue flora samples from 18- to 48-month-old children and periodontal status of their mothers. Oral Microbiol Immunol. 2002;17(1):55–9.
Diaz PI, Chalmers NI, Rickard AH, Kong C, Milburn CL, Palmer RJ Jr, et al. Molecular characterization of subject-specific oral microflora during initial colonization of enamel. Appl Environ Microbiol. 2006;72(4):2837–48.
Colombo AP, Boches SK, Cotton SL, Goodson JM, Kent R, Haffajee AD, et al. Comparisons of subgingival microbial profiles of refractory periodontitis, severe periodontitis, and periodontal health using the human oral microbe identification microarray. J Periodontol. 2009;80(9):1421–32.
Kroes I, Lepp PW, Relman DA. Bacterial diversity within the human subgingival crevice. Proc Natl Acad Sci U S A. 1999;96(25):14547–52.
Kumar PS, Griffen AL, Moeschberger ML, Leys EJ. Identification of candidate periodontal pathogens and beneficial species by quantitative 16S clonal analysis. J Clin Microbiol. 2005;43(8):3944–55.
Moore WE, Moore LV. The bacteria of periodontal diseases. Periodontol 2000. 1994;5:66–77.
Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, et al. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183(12):3770–83.
Quirynen M, Vogels R, Pauwels M, Haffajee AD, Socransky SS, Uzel NG, et al. Initial subgingival colonization of ‘pristine’ pockets. J Dent Res. 2005;84(4):340–4.
Eren AM, Borisy GG, Huse SM, Mark Welch JL. Oligotyping analysis of the human oral microbiome. Proc Natl Acad Sci U S A. 2014;111(28):E2875–84.
Simon-Soro A, Tomas I, Cabrera-Rubio R, Catalan MD, Nyvad B, Mira A. Microbial geography of the oral cavity. J Dent Res. 2013;92(7):616–21.
Abiko Y, Sato T, Mayanagi G, Takahashi N. Profiling of subgingival plaque biofilm microflora from periodontally healthy subjects and from subjects with periodontitis using quantitative real-time PCR. J Periodontal Res. 2010;45(3):389–95.
Abusleme L, Dupuy AK, Dutzan N, Silva N, Burleson JA, Strausbaugh LD, et al. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. ISME J. 2013;7(5):1016–25.
Li Y, He J, He Z, Zhou Y, Yuan M, Xu X, et al. Phylogenetic and functional gene structure shifts of the oral microbiomes in periodontitis patients. ISME J. 2014;8(9):1879–91.
Griffen AL, Beall CJ, Campbell JH, Firestone ND, Kumar PS, Yang ZK, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. ISME J. 2012;6(6):1176–85.
Valm AM, Mark Welch JL, Rieken CW, Hasegawa Y, Sogin ML, Oldenbourg R, et al. Systems-level analysis of microbial community organization through combinatorial labeling and spectral imaging. Proc Natl Acad Sci U S A. 2011;108(10):4152–7.
Mark Welch JL, Rossetti BJ, Rieken CW, Dewhirst FE, Borisy GG. Biogeography of a human oral microbiome at the micron scale. Proc Natl Acad Sci U S A. 2016;113(6):E791–800.
Wright CJ, Burns LH, Jack AA, Back CR, Dutton LC, Nobbs AH, et al. Microbial interactions in building of communities. Mol Oral Microbiol. 2013;28(2):83–101.
Kuboniwa M, Lamont RJ. Subgingival biofilm formation. Periodontol 2000. 2010;52(1):38–52.
Kuboniwa M, Houser JR, Hendrickson EL, Wang Q, Alghamdi SA, Sakanaka A, et al. Metabolic crosstalk regulates Porphyromonas gingivalis colonization and virulence during oral polymicrobial infection. Nat Microbiol. 2017;2(11):1493–9.
Liu C, Miller DP, Wang Y, Merchant M, Lamont RJ. Structure-function aspects of the Porphyromonas gingivalis tyrosine kinase Ptk1. Mol Oral Microbiol. 2017;32(4):314–23.
Maeda K, Tribble GD, Tucker CM, Anaya C, Shizukuishi S, Lewis JP, et al. A Porphyromonas gingivalis tyrosine phosphatase is a multifunctional regulator of virulence attributes. Mol Microbiol. 2008;69(5):1153–64.
Daep CA, Novak EA, Lamont RJ, Demuth DR. Structural dissection and in vivo effectiveness of a peptide inhibitor of Porphyromonas gingivalis adherence to Streptococcus gordonii. Infect Immun. 2011;79(1):67–74.
Whitmore SE, Lamont RJ. The pathogenic persona of community-associated oral streptococci. Mol Microbiol. 2011;81(2):305–14.
Enersen M, Nakano K, Amano A. Porphyromonas gingivalis fimbriae. J Oral Microbiol. 2013;5: https://doi.org/10.3402/jom.v5i0.20265.
Lee JY, Miller DP, Wu L, Casella CR, Hasegawa Y, Lamont RJ. Maturation of the Mfa1 fimbriae in the oral pathogen Porphyromonas gingivalis. Front Cell Infect Microbiol. 2018;8:137.
Hall M, Hasegawa Y, Yoshimura F, Persson K. Structural and functional characterization of shaft, anchor, and tip proteins of the Mfa1 fimbria from the periodontal pathogen Porphyromonas gingivalis. Sci Rep. 2018;8(1):1793.
Alaei S, Park J, Walker S, Thanasi D. Peptide-based inhibitors of fimbrial biogenesis in Porphyromonas gingivalis. Infect Immun. 2019;87(3):e00750–18.
Brooks W, Demuth DR, Gil S, Lamont RJ. Identification of a Streptococcus gordonii SspB domain that mediates adhesion to Porphyromonas gingivalis. Infect Immun. 1997;65(9):3753–8.
Demuth DR, Irvine DC, Costerton JW, Cook GS, Lamont RJ. Discrete protein determinant directs the species-specific adherence of Porphyromonas gingivalis to oral streptococci. Infect Immun. 2001;69(9):5736–41.
Daep CA, James DM, Lamont RJ, Demuth DR. Structural characterization of peptide-mediated inhibition of Porphyromonas gingivalis biofilm formation. Infect Immun. 2006;74(10):5756–62.
Daep CA, Lamont RJ, Demuth DR. Interaction of Porphyromonas gingivalis with oral streptococci requires a motif that resembles the eukaryotic nuclear receptor box protein-protein interaction domain. Infect Immun. 2008;76(7):3273–80.
Forsgren N, Lamont RJ, Persson K. Two intramolecular isopeptide bonds are identified in the crystal structure of the Streptococcus gordonii SspB C-terminal domain. J Mol Biol. 2010;397(3):740–51.
Kalia P, Jain A, Radha Krishnan R, Demuth DR, Steinbach-Rankins JM. Peptide-modified nanoparticles inhibit formation of Porphyromonas gingivalis biofilms with Streptococcus gordonii. Int J Nanomedicine. 2017;12:4553–62.
Mahmoud MY, Demuth DR, Steinbach-Rankins JM. BAR-encapsulated nanoparticles for the inhibition and disruption of Porphyromonas gingivalis-Streptococcus gordonii biofilms. J Nanobiotechnol. 2018;16(1):69.
Patil PC, Tan J, Demuth DR, Luzzio FA. 1,2,3-Triazole-based inhibitors of Porphyromonas gingivalis adherence to oral streptococci and biofilm formation. Bioorg Med Chem. 2016;24(21):5410–7.
Tan J, Patil PC, Luzzio FA, Demuth DR. In vitro and in vivo activity of peptidomimetic compounds that target the periodontal pathogen Porphyromonas gingivalis. Antimicrob Agents Chemother. 2018;62(7): e00400–18.
Grenier D. Antagonistic effect of oral bacteria towards Treponema denticola. J Clin Microbiol. 1996;34(5):1249–52.
Duan D, Scoffield JA, Zhou X, Wu H. Fine-tuned production of hydrogen peroxide promotes biofilm formation of Streptococcus parasanguinis by a pathogenic cohabitant Aggregatibacter actinomycetemcomitans. Environ Microbiol. 2016;18:40023–4036.
Stacy A, Everett J, Jorth P, Trivedi U, Rumbaugh KP, Whiteley M. Bacterial fight-and-flight responses enhance virulence in a polymicrobial infection. Proc Natl Acad Sci U S A. 2014;111(21):7819–24.
Duran-Pinedo AE, Baker VD, Frias-Lopez J. The periodontal pathogen Porphyromonas gingivalis induces expression of transposases and cell death of Streptococcus mitis in a biofilm model. Infect Immun. 2014;82(8):3374–82.
Xie H, Cook GS, Costerton JW, Bruce G, Rose TM, Lamont RJ. Intergeneric communication in dental plaque biofilms. J Bacteriol. 2000;182(24):7067–9.
Xie H, Hong J, Sharma A, Wang BY. Streptococcus cristatus ArcA interferes with Porphyromonas gingivalis pathogenicity in mice. J Periodontal Res. 2012;47(5):578–83. https://doi.org/10.1111/j.600-0765.2012.01469.x.
Wang BY, Wu J, Lamont RJ, Lin X, Xie H. Negative correlation of distributions of Streptococcus cristatus and Porphyromonas gingivalis in subgingival plaque. J Clin Microbiol. 2009;47(12):3902–6.
Xie H, Lin X, Wang BY, Wu J, Lamont RJ. Identification of a signalling molecule involved in bacterial intergeneric communication. Microbiology. 2007;153(Pt 10):3228–34.
Curran TM, Lieou J, Marquis RE. Arginine deiminase system and acid adaptation of oral streptococci. Appl Environ Microbiol. 1995;61(12):4494–6.
Cugini C, Stephens DN, Nguyen D, Kantarci A, Davey ME. Arginine deiminase inhibits Porphyromonas gingivalis surface attachment. Microbiology. 2013;159(Pt 2):275–85.
Zeng L, Dong Y, Burne RA. Characterization of cis-acting sites controlling arginine deiminase gene expression in Streptococcus gordonii. J Bacteriol. 2006;188(3):941–9.
Lin X, Lamont RJ, Wu J, Xie H. Role of differential expression of streptococcal arginine deiminase in inhibition of fimA expression in Porphyromonas gingivalis. J Bacteriol. 2008;190(12):4367–71.
Christopher AB, Arndt A, Cugini C, Davey ME. A streptococcal effector protein that inhibits Porphyromonas gingivalis biofilm development. Microbiology. 2010;156(Pt 11):3469–77.
Ho MH, Lamont RJ, Xie H. A novel peptidic inhibitor derived from Streptococcus cristatus ArcA attenuates virulence potential of Porphyromonas gingivalis. Sci Rep. 2017;7(1):16217.
Ho MH, Lamont RJ, Chazin WJ, Chen H, Young DF, Kumar P, et al. Characterization and development of SAPP as a specific peptidic inhibitor that targets Porphyromonas gingivalis. Mol Oral Microbiol. 2018;33(6):430–9.
Tribble GD, Lamont RJ. Bacterial invasion of epithelial cells and spreading in periodontal tissue. Periodontol 2000. 2010;52(1):68–83.
Yilmaz O, Watanabe K, Lamont RJ. Involvement of integrins in fimbriae-mediated binding and invasion by Porphyromonas gingivalis. Cell Microbiol. 2002;4(5):305–14.
Takeuchi H, Hirano T, Whitmore SE, Morisaki I, Amano A, Lamont RJ. The serine phosphatase SerB of Porphyromonas gingivalis suppresses IL-8 production by dephosphorylation of NF-kappaB RelA/p65. PLoS Pathog. 2013;9(4):e1003326.
Jauregui CE, Wang Q, Wright CJ, Takeuchi H, Uriarte SM, Lamont RJ. Suppression of T-cell chemokines by Porphyromonas gingivalis. Infect Immun. 2013;81(7):2288–95.
Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interf Cytokine Res. 2009;29(6):313–26.
Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol. 2010;8(7):481–90.
Wirth C, Meyer-Klaucke W, Pattus F, Cobessi D. From the periplasmic signaling domain to the extracellular face of an outer membrane signal transducer of Pseudomonas aeruginosa: crystal structure of the ferric pyoverdine outer membrane receptor. J Mol Biol. 2007;368(2):398–406.
Goulas T, Ferrer IG, Hutcherson JA, Potempa BA, Potempa J, Scott DA, et al. Structure of RagB, a major immunodominant outer-membrane surface receptor antigen of Porphyromonas gingivalis. Mol Oral Microbiol. 2016;31(6):472–85.
Hutcherson JA, Bagaitkar J, Nagano K, Yoshimura F, Wang H, Scott DA. Porphyromonas gingivalis RagB is a proinflammatory signal transducer and activator of transcription 4 agonist. Mol Oral Microbiol. 2015;30(3):242–52.
Nagano K, Murakami Y, Nishikawa K, Sakakibara J, Shimozato K, Yoshimura F. Characterization of RagA and RagB in Porphyromonas gingivalis: study using gene-deletion mutants. J Med Microbiol. 2007;56(Pt 11):1536–48.
Jepsen K, Jepsen S. Antibiotics/antimicrobials: systemic and local administration in the therapy of mild to moderately advanced periodontitis. Periodontol 2000. 2016;71(1):82–112.
Santos RS, Macedo RF, Souza EA, Soares RS, Feitosa DS, Sarmento CF. The use of systemic antibiotics in the treatment of refractory periodontitis: a systematic review. J Am Dent Assoc. 2016;147(7):577–85.
Walters J, Lai PC. Should antibiotics be prescribed to treat chronic periodontitis? Dent Clin N Am. 2015;59(4):919–33.
Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota? Nat Rev Microbiol. 2009;7(12):887–94.
Brook I. Antibiotic resistance of oral anaerobic bacteria and their effect on the management of upper respiratory tract and head and neck infections. Semin Respir Infect. 2002;17(3):195–203.
Claffey N, Polyzois I, Ziaka P. An overview of nonsurgical and surgical therapy. Periodontol 2000. 2004;36:35–44.
Fisher S, Kells L, Picard JP, Gelskey SC, Singer DL, Lix L, et al. Progression of periodontal disease in a maintenance population of smokers and non-smokers: a 3-year longitudinal study. J Periodontol. 2008;79(3):461–8.
Uzel NG, Teles FR, Teles RP, Song XQ, Torresyap G, Socransky SS, et al. Microbial shifts during dental biofilm re-development in the absence of oral hygiene in periodontal health and disease. J Clin Periodontol. 2011;38(7):612–20.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Xie, H., Lamont, R.J. (2020). Bacterial Peptides Targeting Periodontal Pathogens in Communities. In: Sahingur, S. (eds) Emerging Therapies in Periodontics. Springer, Cham. https://doi.org/10.1007/978-3-030-42990-4_11
Download citation
DOI: https://doi.org/10.1007/978-3-030-42990-4_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-42989-8
Online ISBN: 978-3-030-42990-4
eBook Packages: MedicineMedicine (R0)