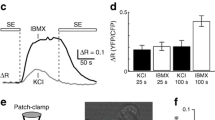
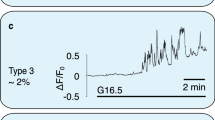
Abstract
Insulin is a key molecule to maintain glucose hemostasis in the body. In pancreatic beta cells, insulin secretion is triggered by Ca2+ and amplified by cAMP/PKA signaling pathway. In the past few years, several studies have shown that these two signaling pathways are coupled with each other, although the causal relationship between them is still obscure. By combining FRET imaging and electrophysiological methods, a recent report confirms the role of Ca2+ to activate cAMP/PKA pathway through adenylyl cyclase 8 (AC8), a Ca2+-stimulated AC isoform. Simultaneous recordings of PKA activity and insulin granule exocytosis suggest the dual roles of Ca2+ in insulin secretion: to trigger acute exocytosis directly and to maintain sustained insulin secretion via cAMP/PKA. Here, we briefly summarize the roles of Ca2+, cAMP/PKA, and adenylyl cyclase in glucose/GLP-1-mediated insulin secretion and the unique method used in these studies.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Ammala C, Ashcroft FM, Rorsman P (1993) Calcium-independent potentiation of insulin release by cyclic AMP in single beta-cells. Nature 363:356–358
Ashcroft FM, Rorsman P (2012) Diabetes mellitus and the beta cell: the last ten years. Cell 148:1160–1171
Augustine GJ, Neher E (1992) Calcium requirements for secretion in bovine chromaffin cells. J Physiol 450:247–271
Chen X, Tomchick DR, Kovrigin E, Arac D, Machius M, Sudhof TC, Rizo J (2002) Three-dimensional structure of the complexin/SNARE complex. Neuron 33:397–409
Dachicourt N, Serradas P, Giroix MH, Gangnerau MN, Portha B (1996) Decreased glucose-induced cAMP and insulin release in islets of diabetic rats: reversal by IBMX, glucagon, GIP. Am J Phys 271:E725–E732
Dos Remedios CG, Miki M, Barden JA (1987) Fluorescence resonance energy transfer measurements of distances in actin and myosin. A critical evaluation. J Muscle Res Cell Motil 8:97–117
Dou H, Wang C, Wu X, Yao L, Zhang X, Teng S, Xu H, Liu B, Wu Q, Zhang Q, Hu M, Wang Y, Wang L, Wu Y, Shang S, Kang X, Zheng L, Zhang J, Raoux M, Lang J, Li Q, Su J, Yu X, Chen L, Zhou Z (2015) Calcium influx activates adenylyl cyclase 8 for sustained insulin secretion in rat pancreatic beta cells. Diabetologia 58:324–333
Dyachok O, Isakov Y, Sagetorp J, Tengholm A (2006) Oscillations of cyclic AMP in hormone-stimulated insulin-secreting beta-cells. Nature 439:349–352
Dyachok O, Idevall-Hagren O, Sagetorp J, Tian G, Wuttke A, Arrieumerlou C, Akusjarvi G, Gylfe E, Tengholm A (2008) Glucose-induced cyclic AMP oscillations regulate pulsatile insulin secretion. Cell Metab 8:26–37
Elangovan M, Day RN, Periasamy A (2002) Nanosecond fluorescence resonance energy transfer-fluorescence lifetime imaging microscopy to localize the protein interactions in a single living cell. J Microsc 205:3–14
Gallegos LL, Kunkel MT, Newton AC (2006) Targeting protein kinase C activity reporter to discrete intracellular regions reveals spatiotemporal differences in agonist-dependent signaling. J Biol Chem 281:30947–30956
Gromada J, Høy F, Renström E, Bokvist K, Eliasson L, Gaboreanu AM, Göpel S, Rorsman P (1999) CaM kinase II-dependent mobilization of secretory granules underlies acetylcholine-induced stimulation of exocytosis in mouse pancreatic B-cells. J Physiol 518:745–759
Guenifi A, Portela-Gomes GM, Grimelius L, Efendic S, Abdel-Halim SM (2000) Adenylyl cyclase isoform expression in non-diabetic and diabetic Goto-Kakizaki (GK) rat pancreas. Evidence for distinct overexpression of type-8 adenylyl cyclase in diabetic GK rat islets. Histochem Cell Biol 113:81–89
Heim R, Tsien RY (1996) Engineering green fluorescent protein for improved brightness, longer wavelengths and fluorescence resonance energy transfer. Curr Biol 6:178–182
Henquin JC, Ishiyama N, Nenquin M, Ravier MA, Jonas JC (2002) Signals and pools underlying biphasic insulin secretion. Diabetes 51:S60–S67
Hodgkin AL, Huxley AF (1952) A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol 117:500–544
Hodson DJ, Mitchell RK, Marselli L, Pullen TJ, Gimeno Brias S, Semplici F, Everett KL, Cooper DM, Bugliani M, Marchetti P, Lavallard V, Bosco D, Piemonti L, Johnson PR, Hughes SJ, Li D, Li WH, Shapiro AM, Rutter GA (2014) Adcy5 couples glucose to insulin secretion in human islets. Diabetes 63:3009–3021
Kasai H, Hatakeyama H, Kishimoto T, Liu TT, Nemoto T, Takahashi N (2005) A new quantitative (two-photon extracellular polar-tracer imaging-based quantification (TEPIQ)) analysis for diameters of exocytic vesicles and its application to mouse pancreatic islets. J Physiol 568:891–903
Landa LR Jr, Harbeck M, Kaihara K, Chepurny O, Kitiphongspattana K, Graf O, Nikolaev VO, Lohse MJ, Holz GG, Roe MW (2005) Interplay of Ca2+ and cAMP signaling in the insulin-secreting MIN6 beta-cell line. J Biol Chem 280:31294–31302
Lawrence GW, Dolly JO (2002) Multiple forms of SNARE complexes in exocytosis from chromaffin cells: effects of Ca2+, MgATP and botulinum toxin type A. J Cell Sci 115:667–673
Leech CA, Castonguay MA, Habener JF (1999) Expression of adenylyl cyclase subtypes in pancreatic beta-cells. Biochem Biophys Res Commun 254:703–706
Lindau M, Neher E (1988) Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflugers Arch 411:137–146
Muller WA, Faloona GR, Aguilar-Parada E, Unger RH (1970) Abnormal alpha-cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med 283:109–115
Neher E, Marty A (1982) Discrete changes of cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc Natl Acad Sci U S A 79:6712–6716
Neher E, Sakaba T (2008) Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron 59:861–872
Neher E, Sakmann B (1976) Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature 260:799–802
Ni Q, Ganesan A, Aye-Han NN, Gao X, Allen MD, Levchenko A, Zhang J (2011) Signaling diversity of PKA achieved via a Ca2+-cAMP-PKA oscillatory circuit. Nat Chem Biol 7:34–40
Ohara-Imaizumi M, Nakamichi Y, Tanaka T, Ishida H, Nagamatsu S (2002) Imaging exocytosis of single insulin secretory granules with evanescent wave microscopy: distinct behavior of granule motion in biphasic insulin release. J Biol Chem 277:3805–3808
Ozaki N, Shibasaki T, Kashima Y, Miki T, Takahashi K, Ueno H, Sunaga Y, Yano H, Matsuura Y, Iwanaga T, Takai Y, Seino S (2000) cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nat Cell Biol 2:805–811
Raoux M, Vacher P, Papin J, Picard A, Kostrzewa E, Devin A, Gaitan J, Limon I, Kas MJ, Magnan C, Lang J (2015) Multilevel control of glucose homeostasis by adenylyl cyclase 8. Diabetologia 58:749–757
Renstrom E, Eliasson L, Rorsman P (1997) Protein kinase A-dependent and -independent stimulation of exocytosis by cAMP in mouse pancreatic B-cells. J Physiol 502(Pt 1):105–118
Roger B, Papin J, Vacher P, Raoux M, Mulot A, Dubois M, Kerr-Conte J, Voy BH, Pattou F, Charpentier G, Jonas JC, Moustaid-Moussa N, Lang J (2011) Adenylyl cyclase 8 is central to glucagon-like peptide 1 signalling and effects of chronically elevated glucose in rat and human pancreatic beta cells. Diabetologia 54:390–402
Rorsman P, Eliasson L, Renström E, Gronmada J, Barg S, Göpel S (2000) The cell physiology of biphasic insulin secretion. News Physiol Sci 15:72–77
Sasmal DK, Lu HP (2014) Single-molecule patch-clamp FRET microscopy studies of NMDA receptor ion channel dynamics in living cells: revealing the multiple conformational states associated with a channel at its electrical off state. J Am Chem Soc 136:12998–13005
Shibasaki T, Takahashi H, Miki T, Sunaga Y, Matsumura K, Yamanaka M, Zhang C, Tamamoto A, Satoh T, Miyazaki J, Seino S (2007) Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc Natl Acad Sci USA 104:19333–19338
Von Rüden L, Neher E (1993) A Ca-dependent early step in the release of catecholamines from adrenal chromaffin cells. Science 262:1061–1065
Wan QF, Dong Y, Yang H, Lou X, Ding J, Xu T (2004) Protein kinase activation increases insulin secretion by sensitizing the secretory machinery to Ca2+. J Gen Physiol 124:653–662
Wang Y, Wu Q, Hu M, Liu B, Chai Z, Huang R, Wang Y, Xu H, Zhou L, Zheng L, Wang C, Zhou Z (2017) Ligand- and voltage-gated Ca2+ channels differentially regulate the mode of vesicular neuropeptide release in mammalian sensory neurons. Sci Signal 10:eaal1683
Willoughby D, Cooper DM (2007) Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev 87:965–1010
Willoughby D, Masada N, Wachten S, Pagano M, Halls ML, Everett KL, Ciruela A, Cooper DM (2010) AKAP79/150 interacts with AC8 and regulates Ca2+-dependent cAMP synthesis in pancreatic and neuronal systems. J Biol Chem 285:20328–20342
Willoughby D, Everett KL, Halls ML, Pacheco J, Skroblin P, Vaca L, Klussmann E, Cooper DM (2012) Direct binding between Orai1 and Ac8 mediates dynamic interplay between Ca2+ and cAMP signaling. Sci Signal 5:Ra29
Zhu D, Zhang Y, Lam PP, Dolai S, Liu Y, Cai EP, Choi D, Schroer SA, Kang Y, Allister EM, Qin T, Wheeler MB, Wang CC, Hong WJ, Woo M, Gaisano HY (2012) Dual role of Vamp8 in regulating insulin exocytosis and islet beta cell growth. Cell Metab 16:238–249
Acknowledgments
We thank Li Zhou, Hongping Huang, Quanfeng Zhang, Younus M. Khan, and Changhe Wang for helpful comments. This work was supported by grants from the National Natural Science Foundation of China (31228010, 31171026, 31100597, 31327901, 31221002, 31330024, 31670843, 31521062, and 31400708) and the National Key Research and Development Program of China (2016YFA0500401).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Key References: See Main List for Reference Details
Key References: See Main List for Reference Details
-
Ammala et al. (1993). The role of cAMP/PKA pathway to potentiate insulin secretion was demonstrated for the first time in pancreatic beta cells.
-
Dou et al. (2015). This paper revealed the dual roles of Ca2+ in regulating insulin secretion, to trigger exocytosis directly, and to replenish pools of insulin granules via the AC-cAMP/PKA pathway.
-
Dyachok et al. (2008). Real-time monitoring of glucose-induced cAMP oscillation in beta cells was achieved in this paper.
-
Ozaki et al. (2000). A Ca2+-cAMP-PKA circuit was studied thoroughly in pancreatic beta cells, by using a unique FRET-based PKA indicator, AKAR3.
-
Von Rüden and Neher (1993). The role of Ca2+ to replenish vesicle pools was first studied in this paper.
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Dou, H., Zhou, Z. (2020). Action Potential-Induced Ca2+ Influx for Both Acute and Sustained Insulin Secretion in Pancreatic Beta Cells. In: Lemos, J., Dayanithi, G. (eds) Neurosecretion: Secretory Mechanisms. Masterclass in Neuroendocrinology, vol 8. Springer, Cham. https://doi.org/10.1007/978-3-030-22989-4_8
Download citation
DOI: https://doi.org/10.1007/978-3-030-22989-4_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-22988-7
Online ISBN: 978-3-030-22989-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)