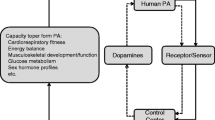
Abstract
Obesity and diabetes have become increasingly prevalent during the past century. Concomitant with this rise, the consumption of trans-fatty acids and processed carbohydrates is likely to have increased and physical activity levels declined. However, the rates at which obesity and diabetes have increased differ across people of varying ethnicities living in the same environment, suggesting the presence of interaction between ethnic-specific factors, such as genes, and changing environments and lifestyles. Quantifying these interactions is difficult because the interaction effect is often small, and precise measurement of lifestyle factors, such as diet and habitual physical activity, is difficult. Conventional interaction studies aim to test whether the magnitude of the association between the lifestyle exposures and the disease outcome is different in those who carry the variant allele at a given locus by comparison with those who do not. Because exercising skeletal muscle is a major site for glucose and lipid metabolism, variants in the genes that are located within muscle and that are up-regulated in response to physical activity present interesting candidates for testing in studies of gene textmultiply physical activity interaction in diabetes. However, numerous methodological limitations seriously hinder attempts to test such hypotheses. This chapter describes (1) a brief review of studies that provide evidence of gene textmultiply physical activity interaction in diabetes (and related traits), (2) functional evidence for interaction between genetic factors and physical activity in metabolic dysregulation, and (3) some common methodological issues that face the study of gene textmultiply environment interaction in human populations.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
Abstract
Wendorf M. Diabetes, the ice free corridor, and the Paleoindian settlement of North America. Am J Phys Anthropol 1989;79(4):503–20.
Prentice AM, Jebb SA. Obesity in Britain: gluttony or sloth? BMJ 1995;311(7002):437–9.
Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet 1997;349(9061):1269–76.
King H, Rewers M. Global estimates for prevalence of diabetes mellitus and impaired glucose tolerance in adults. WHO Ad Hoc Diabetes Reporting Group. Diabetes Care 1993;16(1):157–77.
Hirschhorn JN, Lohmueller K, Byrne E, Hirschhorn K. A comprehensive review of genetic association studies. Genet Med 2002;4(2):45–61.
Franks PW, Barroso I, Luan JA, et al. PGC-1alpha genotype modifies the association of volitional energy expenditure with VO2max. Med Sci Sports Exerc 2003;35(12):1998–2004.
Franks PW, Bhattacharyya S, Luan J, et al. Association between physical activity and blood pressure is modified by variants in the G-protein coupled receptor 10. Hypertension. 2004;43(2):224–8.
Franks PW, Knowler WC, Nair S, et al. Interaction between an 11betaHSD1 gene variant and birth era modifies the risk of hypertension in Pima Indians. Hypertension 2004;44(5):681–8.
Franks PW, Luan J, Browne PO, et al. Does peroxisome proliferator-activated receptor gamma genotype (Pro12ala) modify the association of physical activity and dietary fat with fasting insulin level? Metabolism 2004;53(1):11–6.
Franks PW, Luan Ja, Barroso I, et al. Variation in the eNOS gene modifies the association between total energy expenditure and glucose intolerance. Diabetes 2005;54(9):2795–801.
Meirhaeghe A, Helbecque N, Cottel D, Amouyel P. Beta2-adrenoceptor gene polymorphism, body weight, and physical activity. Lancet 1999;353(9156):896.
Li T, Stefansson H, Gudfinnsson E, et al. Identification of a novel neuregulin 1 at-risk haplotype in Han schizophrenia Chinese patients, but no association with the Icelandic/Scottish risk haplotype. Mol Psychiatr 2004;9(7):698–704.
Stefansson H, Sarginson J, Kong A, et al. Association of neuregulin 1 with schizophrenia confirmed in a Scottish population. Am J Hum Genet 2003;72(1):83–7.
Stefansson H, Sigurdsson E, Steinthorsdottir V, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet 2002;71(4):877–92.
Burchfiel CM, Sharp DS, Curb JD, et al. Physical activity and incidence of diabetes: the Honolulu Heart Program. Am J Epidemiol 1995;141(4):360–8.
Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA 2001;286(10):1218–27.
Kujala UM, Kaprio J, Sarna S, Koskenvuo M. Relationship of leisure-time physical activity and mortality: the Finnish twin cohort. JAMA 1998;279(6):440–4.
U.S. Department of Health and Human Services, Health Resources and Services Administration, Maternal and Child Health Bureau. Child Health USA 2004. Rockville, Maryland: U.S. Department of Health and Human Services; 2004.
Pols MA, Peeters PH, Twisk JW, Kemper HC, Grobbee DE. Physical activity and cardiovascular disease risk profile in women. Am J Epidemiol 1997;146(4):322–8.
Teran-Garcia M, Rankinen T, Koza RA, Rao DC, Bouchard C. Endurance training-induced changes in insulin sensitivity and gene expression. Am J Physiol Endocrinol Metab 2005;288(6):E1168–78.
Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20(4):537–44.
Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344(18):1343–50.
Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346(6):393–403.
Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 2006;49(2):289–97.
Bouchard C, Rankinen T. Individual differences in response to regular physical activity. Med Sci Sports Exerc 2001;33(Suppl 6):S446–51.
Franks PW, Mesa JL, Harding AH, Wareham NJ. Gene-lifestyle interaction on risk of type 2 diabetes. Nutr Metab Cardiovasc Dis2007;17(2):104–24.
Bernstein MS, Costanza MC, James RW, et al. Physical activity may modulate effects of ApoE genotype on lipid profile. Arterioscler Thromb Vasc Biol 2002;22(1):133–40.
Corella D, Guillen M, Saiz C, et al. Environmental factors modulate the effect of the APOE genetic polymorphism on plasma lipid concentrations: ecogenetic studies in a Mediterranean Spanish population. Metabolism 2001;50(8):936–44.
Senti M, Elosua R, Tomas M, et al. Physical activity modulates the combined effect of a common variant of the lipoprotein lipase gene and smoking on serum triglyceride levels and high-density lipoprotein cholesterol in men. Hum Genet 2001;109(4):385–92.
Taimela S, Lehtimaki T, Porkka KV, Rasanen L, Viikari JS. The effect of physical activity on serum total and low-density lipoprotein cholesterol concentrations varies with apolipoprotein E phenotype in male children and young adults: The Cardiovascular Risk in Young Finns Study. Metabolism 1996;45(7):797–803.
McCole SD, Shuldiner AR, Brown MD, et al. Beta2- and beta3-adrenergic receptor polymorphisms and exercise hemodynamics in postmenopausal women. J Appl Physiol 2004;96(2):526–30.
Otabe S, Clement K, Dina C, et al. A genetic variation in the 5’ flanking region of the UCP3 gene is associated with body mass index in humans in interaction with physical activity. Diabetologia 2000;43(2):245–9.
Boer JM, Kuivenhoven JA, Feskens EJ, et al. Physical activity modulates the effect of a lipoprotein lipase mutation (D9N) on plasma lipids and lipoproteins. Clin Genet 1999;56(2):158–63.
Ortlepp JR, Metrikat J, Albrecht M, von Korff A, Hanrath P, Hoffmann R. The vitamin D receptor gene variant and physical activity predicts fasting glucose levels in healthy young men. Diabet Med 2003;20(6):451–4.
Rauramaa R, Vaisanen S, Nissinen A, et al. Physical activity, fibrinogen plasma level and gene polymorphisms in postmenopausal women. Thromb Haemost 1997;78(2):840–4.
Kimura T, Yokoyama T, Matsumura Y, et al. NOS3 genotype-dependent correlation between blood pressure and physical activity. Hypertension 2003;41(2):355–60.
Corbalan MS, Marti A, Forga L, Martinez-Gonzalez MA, Martinez JA. The 27Glu polymorphism of the beta2-adrenergic receptor gene interacts with physical activity influencing obesity risk among female subjects. Clin Genet 2002;61(4):305–7.
Pisciotta L, Cantafora A, Piana A, et al. Physical activity modulates effects of some genetic polymorphisms affecting cardiovascular risk in men aged over 40 years. Nutr Metab Cardiovasc Dis 2003;13(4):202–10.
Hokanson JE, Kamboh MI, Scarboro S, Eckel RH, Hamman RF. Effects of the hepatic lipase gene and physical activity on coronary heart disease risk. Am J Epidemiol 2003;158(9):836–43.
Berentzen T, Dalgaard LT, Petersen L, Pedersen O, Sorensen TI. Interactions between physical activity and variants of the genes encoding uncoupling proteins -2 and -3 in relation to body weight changes during a 10-y follow-up. Int J Obes (Lond) 2005;29(1):93–9.
Bouchard C, Leon AS, Rao DC, Skinner JS, Wilmore JH, Gagnon J. The HERITAGE family study. Aims, design, and measurement protocol. Med Sci Sports Exerc 1995 May;27(5):721–9.
Lakka TA, Rankinen T, Weisnagel SJ, et al. Leptin and leptin receptor gene polymorphisms and changes in glucose homeostasis in response to regular exercise in nondiabetic individuals: the HERITAGE family study. Diabetes 2004;53(6):1603–8.
Lakka TA, Rankinen T, Weisnagel SJ, et al. A quantitative trait locus on 7q31 for the changes in plasma insulin in response to exercise training: the HERITAGE Family Study. Diabetes 2003;52(6):1583–7.
Rankinen T, Rice T, Perusse L, et al. NOS3 Glu298Asp genotype and blood pressure response to endurance training: the HERITAGE family study. Hypertension 2000;36(5):885–9.
Lindi VI, Uusitupa MI, Lindstrom J, et al. Association of the Pro12Ala polymorphism in the PPAR-gamma2 gene with 3-year incidence of type 2 diabetes and body weight change in the Finnish Diabetes Prevention Study. Diabetes 2002;51(8):2581–6.
Kubaszek A, Pihlajamaki J, Komarovski V, et al. Promoter polymorphisms of the TNF-alpha (G-308A) and IL-6 (C-174G) genes predict the conversion from impaired glucose tolerance to type 2 diabetes: the Finnish Diabetes Prevention Study. Diabetes 2003;52(7):1872–6.
Todorova B, Kubaszek A, Pihlajamaki J, et al. The G-250A promoter polymorphism of the hepatic lipase gene predicts the conversion from impaired glucose tolerance to type 2 diabetes mellitus: the Finnish Diabetes Prevention Study. J Clin Endocrinol Metab 2004;89(5):2019–23.
The Diabetes Prevention Program Research Group. The Diabetes Prevention Program: recruitment methods and results. Control Clin Trials 2002;23(2):157–71.
Florez JC, Jablonski KA, Bayley N, et al. TCF7L2 polymorphisms and progression to diabetes in the Diabetes Prevention Program. N Engl J Med 2006;355(3):241–50.
Florez JC, Jablonski KA, Kahn SE, et al. Type 2 diabetes-associated missense polymorphisms KCNJ11 E23K and ABCC8 A1369S influence progression to diabetes and response to interventions in the Diabetes Prevention Program. Diabetes 2007;56(2):531–6.
Florez JC, Jablonski KA, Sun MW, et al. Effects of the type 2 diabetes-associated PPARG P12A polymorphism on progression to diabetes and response to troglitazone. J Clin Endocrinol Metab 2007;92(4):1502–9.
Franks PW, Jablonski KA, Florez JC, et al. The Pro12Ala variant at the PPARG gene and change in obesity-related traits in the Diabetes Prevention Program. Diabetologia (in press).
DeFronzo RA. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidaemia and atherosclerosis. Neth J Med 1997;50(5):191–7.
Wasserman D. An overview of muscle glucose uptake during exercise: sites of regulation. London: Plenum; 1998.
Shulman GI. Cellular mechanisms of insulin resistance in humans. Am J Cardiol 1999;84(1A):3J–10J.
World Health Organisation WHO Expert Committee on Diabetes. Second Report. Geneva: World Health Organisation; 1980.
Richter EA, Nielsen JN, Jorgensen SB, Frosig C, Wojtaszewski JF. Signalling to glucose transport in skeletal muscle during exercise. Acta Physiol Scand 2003;178(4):329–35.
Soyal S, Krempler F, Oberkofler H, Patsch W. PGC-1alpha: a potent transcriptional cofactor involved in the pathogenesis of type 2 diabetes. Diabetologia 2006;49(7):1477–88.
Mootha VK, Lindgren CM, Eriksson KF, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 2003;34(3):267–73.
Semple RK, Crowley VC, Sewter CP, et al. Expression of the thermogenic nuclear hormone receptor coactivator PGC-1alpha is reduced in the adipose tissue of morbidly obese subjects. Int J Obes Relat Metab Disord 2004;28(1):176–9.
Baar K, Wende A, Jones TE, et al. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J 2002;16(14):1879–86.
Goto M, Terada S, Kato M, et al. cDNA Cloning and mRNA analysis of PGC-1 in epitrochlearis muscle in swimming-exercised rats. Biochem Biophys Res Commun 2000;274(2):350–4.
Lin J, Wu H, Tarr PT, et al. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 2002;418(6899):797–801.
Terada S, Goto M, Kato M, Kawanaka K, Shimokawa T, Tabata I. Effects of low-intensity prolonged exercise on PGC-1 mRNA expression in rat epitrochlearis muscle. Biochem Biophys Res Commun 2002;296(2):350–4.
Wu H, Kanatous SB, Thurmond FA, et al. Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science 2002;296(5566):349–52.
Terada S, Tabata I. Effects of acute bouts of running and swimming exercise on PGC-1alpha protein expression in rat epitrochlearis and soleus muscle. Am J Physiol Endocrinol Metab 2004;286(2): E208–16.
Konrad D, Somwar R, Sweeney G, et al. The antihyperglycemic drug alpha-lipoic acid stimulates glucose uptake via both GLUT4 translocation and GLUT4 activation: potential role of p38 mitogen-activated protein kinase in GLUT4 activation. Diabetes 2001;50(6):1464–71.
Richter EA, Nielsen JN, Jorgensen SB, Frosig C, Birk JB, Wojtaszewski JF. Exercise signalling to glucose transport in skeletal muscle. Proc Nutr Soc 2004;63(2):211–6.
Yonemitsu S, Nishimura H, Shintani M, et al. Troglitazone induces GLUT4 translocation in L6 myotubes. Diabetes 2001;50(5):1093–101.
Michael LF, Wu Z, Cheatham RB, et al. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci USA 2001;98(7):3820–5.
Handschin C, Rhee J, Lin J, Tarr PT, Spiegelman BM. An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1alpha expression in muscle. Proc Natl Acad Sci USA 2003;100(12):7111–6.
Herzig S, Hedrick S, Morantte I, Koo SH, Galimi F, Montminy M. CREB controls hepatic lipid metabolism through nuclear hormone receptor PPAR-gamma. Nature 2003;426(6963):190–3.
Tsao TS, Li J, Chang KS, et al. Metabolic adaptations in skeletal muscle overexpressing GLUT4: effects on muscle and physical activity. FASEB J 2001;15(6):958–69.
Zisman A, Peroni OD, Abel ED, et al. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat Med 2000;6(8):924–8.
Gibbs EM, Stock JL, McCoid SC, et al. Glycemic improvement in diabetic db/db mice by overexpression of the human insulin-regulatable glucose transporter (GLUT4). J Clin Invest 1995;95(4):1512–8.
Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol 2003;546(Pt 3):851–8.
Norrbom J, Sundberg CJ, Ameln H, Kraus WE, Jansson E, Gustafsson T. PGC-1alpha mRNA expression is influenced by metabolic perturbation in exercising human skeletal muscle. J Appl Physiol 2004;96(1):189–94.
Short KR, Vittone JL, Bigelow ML, et al. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes 2003;52(8):1888–96.
Russell AP, Feilchenfeldt J, Schreiber S, et al. Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-gamma coactivator-1 and peroxisome proliferator-activated receptor-alpha in skeletal muscle. Diabetes 2003;52(12):2874–81.
Tunstall RJ, Mehan KA, Wadley GD, et al. Exercise training increases lipid metabolism gene expression in human skeletal muscle. Am J Physiol Endocrinol Metab 2002;283(1):E66–72.
Barroso I, Luan J, Sandhu M, Franks PW, Crowley V, Schafer A, et al. Meta-analysis of the Gly482Ser variant in PPARGC1A in type 2 diabetes and related phenotypes. Diabetologia 2006;49(3):501–5.
Ling C, Poulsen P, Carlsson E, et al. Multiple environmental and genetic factors influence skeletal muscle PGC-1alpha and PGC-1beta gene expression in twins. J Clin Invest 2004;114(10):1518–26.
Lucia A, Gomez-Gallego F, Barroso I, et al. PPARGC1A genotype (Gly482Ser) predicts exceptional endurance capacity in European men. J Appl Physiol 2005;99(1):344–8.
Kempen KP, Saris WH, Westerterp KR. Energy balance during an 8-wk energy-restricted diet with and without exercise in obese women. Am J Clin Nutr 1995;62(4):722–9.
Van Etten LM, Westerterp KR, Verstappen FT, Boon BJ, Saris WH. Effect of an 18-wk weight-training program on energy expenditure and physical activity. J Appl Physiol 1997;82(1):298–304.
Wong MY, Day NE, Luan JA, Chan KP, Wareham NJ. The detection of gene-environment interaction for continuous traits: should we deal with measurement error by bigger studies or better measurement? Int J Epidemiol 2003;32(1):51–7.
Montoye H, Kemper H, Saris WHS, Washburn R. Measuring Phsyical Activity and Energy Expenditure. Champaign, IL: Human Kinetics; 1996.
Hanson RL, Pratley RE, Bogardus C, et al. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. Am J Epidemiol 2000;151(2):190–8.
Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22(9):1462–70.
Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet 1962;14:353–62.
Musani SK, Shriner D, Liu N, et al. Detection of gene x gene interactions in genome-wide association studies of human population data. Hum Hered 2007;63(2):67–84.
Steemers FJ, Gunderson KL. Whole genome genotyping technologies on the BeadArray platform. Biotechnol J 2007;2(1):41–9.
Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science 2005;308(5720):385–9.
Helgadottir A, Thorleifsson G, Manolescu A, et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science 2007;316(5830):1491–3.
McPherson R, Pertsemlidis A, Kavaslar N, et al. A common allele on chromosome 9 associated with coronary heart disease. Science 2007;316(5830):1488–91.
Saxena R, Voight BF, Lyssenko V, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 2007;316(5829):1331–6.
Scott LJ, Mohlke KL, Bonnycastle LL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 2007;316(5829):1341–5.
Zeggini E, Weedon MN, Lindgren CM, et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 2007;316(5829):1336–41.
Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 2007;447(7145):661–78.
Chanock SJ, Manolio T, Boehnke M, et al. Replicating genotype-phenotype associations. Nature 2007;447(7145):655–60.
Heck AL, Barroso CS, Callie ME, Bray MS. Gene-nutrition interaction in human performance and exercise response. Nutrition 2004;20(7–8):598–602.
Risch N, Merikangas K. The future of genetic studies of complex human diseases. Science 1996;273(5281):1516–7.
Wang WY, Barratt BJ, Clayton DG, Todd JA. Genome-wide association studies: theoretical and practical concerns. Nat Rev Genet 2005;6(2):109–18.
The International HapMap Project. Nature 2003;426(6968):789–96.
A haplotype map of the human genome. Nature 2005;437(7063):1299–320.
Crawford DC, Nickerson DA. Definition and clinical importance of haplotypes. Annu Rev Med 2005;56:303–20.
Neale BM, Sham PC. The future of association studies: gene-based analysis and replication. Am J Hum Genet 2004;75(3):353–62.
Elston RC, Anne Spence M. Advances in statistical human genetics over the last 25 years. Stat Med 2006;25(18):3049–80.
Heidema AG, Boer JM, Nagelkerke N, Mariman EC, van der AD, Feskens EJ. The challenge for genetic epidemiologists: how to analyze large numbers of SNPs in relation to complex diseases. BMC Genet 2006;7:23.
Nelson MR, Kardia SL, Ferrell RE, Sing CF. A combinatorial partitioning method to identify multilocus genotypic partitions that predict quantitative trait variation. Genome Res 2001;11(3): 458–70.
Ritchie MD, Hahn LW, Roodi N, et al. Multifactor-dimensionality reduction reveals high-order interactions among estrogen-metabolism genes in sporadic breast cancer. Am J Hum Genet 2001;69(1): 138–47.
Thornton-Wells TA, Moore JH, Haines JL. Genetics, statistics and human disease: analytical retooling for complexity. Trends Genet 2004;20(12):640–7.
Lou XY, Chen GB, Yan L, et al. A generalized combinatorial approach for detecting gene-by-gene and gene-by-environment interactions with application to nicotine dependence. Am J Hum Genet 2007;80(6):1125–37.
Cho YM, Ritchie MD, Moore JH, et al. Multifactor-dimensionality reduction shows a two-locus interaction associated with Type 2 diabetes mellitus. Diabetologia 2004;47(3):549–54.
Rebbeck TR, Spitz M, Wu X. Assessing the function of genetic variants in candidate gene association studies. Nat Rev Genet 2004;5(8):589–97.
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2007 Humana Press Inc.
About this chapter
Cite this chapter
Franks, P.W., Roth, S.M. (2007). Interaction Between Physical Activity and Genetic Factors in Complex Metabolic Disease. In: Donohoue, P.A. (eds) Energy Metabolism and Obesity. Contemporary Endocrinology. Humana Press. https://doi.org/10.1007/978-1-60327-139-4_9
Download citation
DOI: https://doi.org/10.1007/978-1-60327-139-4_9
Publisher Name: Humana Press
Print ISBN: 978-1-58829-671-9
Online ISBN: 978-1-60327-139-4
eBook Packages: MedicineMedicine (R0)