Abstract
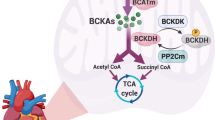
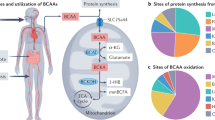
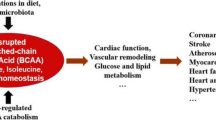
Branched-Chain Amino Acids (BCAA) are essential amino acids for protein synthesis and also serve as critical signaling molecules for cellular growth and metabolic regulations. The homeostasis of BCAA is regulated by food uptake and intrinsic catabolic activities. Genetic defects in BCAA catabolic pathways can cause cardiomyopathies and suppressed BCAA catabolic activities are observed in common forms of human heart diseases. However, the cellular and molecular mechanisms of BCAA function and its misregulation under pathological stress in heart diseases remain to be explored. Nevertheless, BCAA catabolic regulation is potential diagnosis and therapeutic target for heart failure.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Baquet A, Lavoinne A, Hue L. Comparison of the effects of various amino acids on glycogen synthesis, lipogenesis and ketogenesis in isolated rat hepatocytes. Biochem J. 1991;273(Pt 1):57–62. PMC:1149878.
Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr. 1984;4:409–54.
Potier M, Darcel N, Tome D. Protein, amino acids and the control of food intake. Curr Opin Clin Nutr Metab Care. 2009;12(1):54–8.
Marc Rhoads J, Wu G. Glutamine, arginine, and leucine signaling in the intestine. Amino Acids. 2009;37(1):111–22.
Avruch J, Long X, Ortiz-Vega S, Rapley J, Papageorgiou A, Dai N. Amino acid regulation of TOR complex 1. Am J Physiol Endocrinol Metab. 2009;296(4):E592–602. PMC:2670622.
Meijer AJ. Amino acid regulation of autophagosome formation. Methods Mol Biol. 2008;445:89–109.
Vary TC, Lynch CJ. Nutrient signaling components controlling protein synthesis in striated muscle. J Nutr. 2007;137(8):1835–43.
Chotechuang N, Azzout-Marniche D, Bos C, Chaumontet C, Gausseres N, Steiler T, Gaudichon C, Tome D. mTOR, AMPK, and GCN2 coordinate the adaptation of hepatic energy metabolic pathways in response to protein intake in the rat. Am J Physiol Endocrinol Metab. 2009;297(6):E1313–23.
Harris RA, Joshi M, Jeoung NH. Mechanisms responsible for regulation of branched-chain amino acid catabolism. Biochem Biophys Res Commun. 2004;313(2):391–6.
Hutson SM, Sweatt AJ, LaNoue KF. Branched-chain amino acid metabolism: implications for establishing safe intakes. J Nutr. 2005;135(6):1557S–64.
Tso S-C, Qi X, Gui W-J, Chuang JL, Morlock LK, Wallace AL, Ahmed K, Laxman S, Campeau PM, Lee BH, Hutson SM, Tu BP, Williams NS, Tambar UK, Wynn RM, Chuang DT. Structure-based design and mechanisms of allosteric inhibitors for mitochondrial branched-chain α-ketoacid dehydrogenase kinase. Proc Natl Acad Sci. 2013;110(24):9728–33.
Herman MA, She P, Peroni OD, Lynch CJ, Kahn BB. Adipose tissue branched chain amino acid (BCAA) metabolism modulates circulating BCAA levels. J Biol Chem. 2010;285(15):11348–56.
Joshi MA, Jeoung NH, Obayashi M, Hattab EM, Brocken EG, Liechty EA, Kubek MJ, Vattem KM, Wek RC, Harris RA. Impaired growth and neurological abnormalities in branched-chain α-keto acid dehydrogenase kinase-deficient mice. Biochem J. 2006;400(1):153–62.
Fuller TF, Ghazalpour A, Aten JE, Drake TA, Lusis AJ, Horvath S. Weighted gene coexpression network analysis strategies applied to mouse weight. Mamm Genome. 2007;18(6–7):463–72.
Zhou M, Lu G, Gao C, Wang Y, Sun H. Tissue-specific and nutrient regulation of the branched-chain α-keto acid dehydrogenase phosphatase, protein phosphatase 2Cm (PP2Cm). J Biol Chem. 2012;287(28):23397–406.
Nishimura J, Masaki T, Arakawa M, Seike M, Yoshimatsu H. Isoleucine prevents the accumulation of tissue triglycerides and upregulates the expression of PPARalpha and uncoupling protein in diet-induced obese mice. J Nutr. 2010;140(3):496–500.
Proud CG. Regulation of mammalian translation factors by nutrients. Eur J Biochem. 2002;269(22):5338–49.
Zhang D, Contu R, Latronico MVG, Zhang J, Rizzi R, Catalucci D, Miyamoto S, Huang K, Ceci M, Gu Y, Dalton ND, Peterson KL, Guan K-L, Brown JH, Chen J, Sonenberg N, Condorelli G. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J Clin Invest. 2010;120(8):2805–16.
Katta A, Kundla S, Kakarla SK, Wu M, Fannin J, Paturi S, Liu H, Addagarla HS, Blough ER. Impaired overload-induced hypertrophy is associated with diminished mTOR signaling in insulin-resistant skeletal muscle of the obese Zucker rat. Am J Physiol Regul Integr Comp Physiol. 2010;299(6):R1666–75.
Meijer AJ, Dubbelhuis PF. Amino acid signalling and the integration of metabolism. Biochem Biophys Res Commun. 2004;313(2):397–403.
Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy Jr WS, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4):311–26.
Xiao F, Huang Z, Li H, Yu J, Wang C, Chen S, Meng Q, Cheng Y, Gao X, Li J, Liu Y, Guo F. Leucine deprivation increases hepatic insulin sensitivity Via GCN2/mTOR/S6K1 and AMPK pathways. Diabetes. 2011;60(3):746–56.
Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, Myer VE, MacKeigan JP, Porter JA, Wang YK, Cantley LC, Finan PM, Murphy LO. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136(3):521–34.
Nishida K, Kyoi S, Yamaguchi O, Sadoshima J, Otsu K. The role of autophagy in the heart. Cell Death Differ. 2009;16(1):31–8.
D’Antona G, Ragni M, Cardile A, Tedesco L, Dossena M, Bruttini F, Caliaro F, Corsetti G, Bottinelli R, Carruba MO, Valerio A, Nisoli E. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 2010;12(4):362–72.
Arn P, Funanage VL. 3-methylglutaconic aciduria disorders: the clinical spectrum increases. J Pediatr Hematol Oncol. 2006;28(2):62–3.
Bowles KR, Bowles NE. Genetics of inherited cardiomyopathies. Expert Rev Cardiovasc Ther. 2004;2(5):683–97.
Draaisma JM, van Kesteren IC, Daniels O, Sengers RC. Dilated cardiomyopathy with 3-methylglutaconic aciduria. Pediatr Cardiol. 1994;15(2):89–90.
Romano S, Valayannopoulos V, Touati G, Jais JP, Rabier D, de Keyzer Y, Bonnet D, de Lonlay P. Cardiomyopathies in propionic aciduria are reversible after liver transplantation. J Pediatr. 2010;156(1):128–34.
Lu G, Sun H, She P, Youn JY, Warburton S, Ping P, Vondriska TM, Cai H, Lynch CJ, Wang Y. Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells. J Clin Invest. 2009;119(6):1678–87.
Lu G, Ren S, Korge P, Choi J, Dong Y, Weiss J, Koehler C, Chen JN, Wang Y. A novel mitochondrial matrix serine/threonine protein phosphatase regulates the mitochondria permeability transition pore and is essential for cellular survival and development. Genes Dev. 2007;21(7):784–96.
Sun H, Lu G, Ren S, Chen J, Wang Y. Catabolism of branched-chain amino acids in heart failure: insights from genetic models. Pediatr Cardiol. 2011;32(3):305–10.
Shah SH, Bain JR, Muehlbauer MJ, Stevens RD, Crosslin DR, Haynes C, Dungan J, Newby LK, Hauser ER, Ginsburg GS, Newgard CB, Kraus WE. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events/CLINICAL PERSPECTIVE. Circ Cardiovasc Genet. 2010;3(2):207–14.
Kato T, Niizuma S, Inuzuka Y, Kawashima T, Okuda J, Tamaki Y, Iwanaga Y, Narazaki M, Matsuda T, Soga T, Kita T, Kimura T, Shioi T. Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Circulation: Heart Failure; 2010.
Héliès-Toussaint C, Moinard C, Rasmusen C, Tabbi-Anneni I, Cynober L, Grynberg A. Aortic banding in rat as a model to investigate malnutrition associated with heart failure. Am J Physiol Regul Integr Comp Physiol. 2005;288(5):R1325–31.
Bing RJ, Siegel A, Vitale A, Balboni F, Sparks E, Taeschler M, Klapper M, Edwards S. Metabolic studies on the human heart in vivo. I Studies on carbohydrate metabolism of the human heart. Am J Med. 1953;15(3):284–96.
Ingwall JS. Energy metabolism in heart failure and remodelling. Cardiovasc Res. 2009;81(3):412–9. PMC:2639129.
Guertl B, Noehammer C, Hoefler G. Metabolic cardiomyopathies. Int J Exp Pathol. 2000;81(6):349–72.
Lee J-H, Gao C, Peng G, Greer C, Ren S, Wang Y, Xiao X. Analysis of transcriptome complexity through RNA sequencing in normal and failing murine hearts/novelty and significance. Circ Res. 2011;109(12):1332–41.
Mochel F, Charles P, Seguin FO, Barritault J, Coussieu C, Perin L, Le Bouc Y, Gervais C, Carcelain G, Vassault A, Feingold J, Rabier D, Durr A. Early energy deficit in Huntington disease: identification of a plasma biomarker traceable during disease progression. PLoS One. 2007;2(7):e647.
Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O’Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17(4):448–53.
Yudkoff M, Daikhin Y, Nissim I, Horyn O, Luhovyy B, Lazarow A. Brain amino acid requirements and toxicity: the example of leucine. J Nutr. 2005;135(6 Suppl):1531S–8.
Saha AK, Xu XJ, Lawson E, Deoliveira R, Brandon AE, Kraegen EW, Ruderman NB. Downregulation of AMPK accompanies leucine- and glucose-induced increases in protein synthesis and insulin resistance in rat skeletal muscle. Diabetes. 2010;59(10):2426–34.
Kim S, Buel G, Blenis J. Nutrient regulation of the mTOR Complex 1 signaling pathway. Mol Cells. 2013;1–11.
Hill JA. Autophagy in cardiac plasticity and disease. Pediatr Cardiol. 2011;32(3):282–9.
Aviv Y, Shaw J, Gang H, Kirshenbaum LA. Regulation of autophagy in the heart: “You Only Live Twice”. Antioxid Redox Signal. 2011;14(11):2245–50.
Gustafsson AB, Gottlieb RA. Autophagy in ischemic heart disease. Circ Res. 2009;104(2):150–8. PMC:2765251.
de Keyzer Y, Valayannopoulos V, Benoist JF, Batteux F, Lacaille F, Hubert L, Chretien D, Chadefeaux-Vekemans B, Niaudet P, Touati G, Munnich A, de Lonlay P. Multiple OXPHOS deficiency in the liver, kidney, heart, and skeletal muscle of patients with methylmalonic aciduria and propionic aciduria. Pediatr Res. 2009;66(1):91–5.
Shestopalov A, Kristal B. Branched chain keto-acids exert biphasic effects on α-ketoglutarate-stimulated respiration in intact rat liver mitochondria. Neurochem Res. 2007;32(4):947–51.
Sgaravatti AM, Rosa RB, Schuck PF, Ribeiro CAJ, Wannmacher CMD, Wyse ATS, Dutra-Filho CS, Wajner M. Inhibition of brain energy metabolism by the [alpha]-keto acids accumulating in maple syrup urine disease. Biochim Biophys Acta. 2003;1639(3):232–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Sun, H., Wang, Y. (2015). Branched Chain Amino Acids in Heart Failure. In: Rajendram, R., Preedy, V., Patel, V. (eds) Branched Chain Amino Acids in Clinical Nutrition. Nutrition and Health. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-1914-7_6
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1914-7_6
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-1913-0
Online ISBN: 978-1-4939-1914-7
eBook Packages: MedicineMedicine (R0)