Abstract
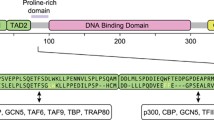
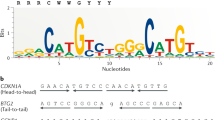
The p53 gene is an important tumor suppressor gene, whose inactivation appears to play a pivotal role in many types of cancer1–3. The p53 protein, encoded by this gene, is a potent sequence-specific transcriptional activator4,5. Binding of the p53 protein to genes which contain consensus p53 binding sites results in a pronounced increase in their transcription rates6–11. Transcriptional activation of relevant target genes, mediated through high affinity sequence-specific DNA binding, is believed to be responsible for many of the biological effects of the wild-type p53 (wt p53) protein. This is supported by the fact that the overwhelming majority of p53 mutations found in human tumors abrogate DNA binding, either by altering direct DNA contact residues or through destabilizing the structure of the DNA binding domain12,13.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
A.J. Levine. 1993. The tumor suppressor genes. Ann. Rev Biochem. 62: 623–651.
L.A. Donehower and A. Bradley. 1993. The tumor suppressor p53. Biochim Biophys Acta. 1155: 181–205.
R. Haffner and M. Oren. 1995. Biochemical properties and biological effects of p53. Curr Op. Genet. Develop.. 5: 84–90.
C. Prives and J.J. Manfredi. 1993. The p53 tumor suppressor protein–meeting review. Genes Dev. 7: 529–534.
B. Vogelstein and K.W. Kinzler. 1992. p53 function and dysfunction. Cell. 70: 523–526.
G. Farmer, J. Bargonetti, H. Zhu, P. Friedman, R. Prywes and C. Prives. 1992. Wild-type p53 activates transcription in vitro. Nature. 358: 83–86.
S.E. Kern, J.A. Pietenpol, S. Thiagalingam, A. Seymour, K.W. Kinzler and B. Vogelstein. 1992. Oncogenic forms of p53 inhibit p53-regulated gene expression. Science. 256: 827–830.
G.P. Zambetti, J. Bargonetti, K. Walker, C. Prives and A.J. Levine. 1992. Wild-type p53 mediates positive regulation of gene expression through a specific DNA sequence element. Genes Dev. 6: 1143–1152.
W.D. Funk, D.T. Pak, R.H. Karas, W.E. Wright and J.W. Shay. 1992. A transcriptionally active dna-binding site for human p53 protein complexes. Mol Cell Biol. 12: 2866–2871.
M.B. Kastan, Q.M. Zhan, W.S. Eldeiry, F. Carrier, T. Jacks, W.V. Walsh, B.S. Plunkett, B. Vogelstein and A.J. Fornace. 1992. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in Ataxia-Telangiectasia. Cell. 71: 587–597.
A. Zauberman, Y. Barak, N. Ragimov, N. Levy and M. Oren. 1993. Sequence-specific DNA binding by p53–identification of target sites and lack of binding to p53-MDM2 complexes. EMBO J. 12: 2799–2808.
C. Prives. 1994. How loops, beta sheets, and alpha helices help us to understand p53. Cell. 78: 543–546.
S. Friend. 1994. p53: a glimpse at the puppet behind the shadow play. Science. 265: 334–335.
W.S. El-Deiry, T. Tokino, V.E. Valculescu, D.B. Levy, R. Parsons, J.M. Trent, D. Lin, W.E. Mercer, K.W. Kinzler and B. Vogelstein. 1993. WAF 1, a potential mediator of p53 tumor suppressor. Cell. 75: 817–825.
J.W. Harper, G.R. Adami, N. Wei, K. Keyomarsi and S.J. Elledge. 1993. The p21 CDK-Interacting protein cipl is a potent inhibitor of gl cyclin-dependent kinases. Cell. 75: 805–816.
Y. Xiong, G.J. Hannon, H. Zhang, D. Casso, R. Kobayashi and D. Beach. 1993. p21 is a universal inhibitor of cyclin kinases. Science. 366: 701–704.
A. Noda, Y. Ning, S.F. Venable, O.M. Pereira-Smith, and J.R. Smith. 1994. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp. Cell Res. 211: 90–98.
V. Dulic, W.K. Kauffmann, S.J. Wilson, T.D. Tlsty, E. Lees, J.W. Harper, S.J. Elledge, and S.I. Reed. 1994. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell 76: 1013–1023.
W.S. EI-Deity, J.W. Harper, P.M. O’Connor, V.E. Verculescu, C.E. Canman, J. Jackson, J.A. Pietenpol, M. Burrell, D.E. Hill, Y. Wang, W.K. Wiman, W.E. Mercer, M.B. Kastan, K.W. Kohn, S.J. Elledge, K.W. Kinzler, and B. Vogelstein. 1994. WAF1/CIP1 is induced in p53-mediated GI arrest and apoptosis. Cancer Res. 54: 1169–1174.
D. Michalovitz, O. Halevy, and M. Oren. 1990. Conditional inhibition of transformation and of cell proliferation by temperature-sensitive mutant of p53. Cell 62: 671–680.
W.E. Mercer, M.T. Shields, M. Amin, G.J. Sauve, E. Appella, S.J. Ullrich and J.W. Romano. 1990. Negative growth regulation in a glioblastoma tumor cell line that conditionally expresses human wild-type p53. Proc. Natl. Acad. Sci. USA. 87: 6166–6170.
S.J. Baker, S. Markowitz, E.R. Fearon, K.V. Willson and B. Vogelstein. 1990. Suppression of human colorectal carcinoma cell growth by wild-type p53. Science. 249: 912–915.
L. Diller, J. Kassel, C.E. Nelson, M.A. Gryka, G. Litwak, M. Gebhardt, B. Bressac, M. Ozturk, S.J. Baker, B. Vogelstein and S.H. Friend. 1990. p53 functions as a cell cycle control protein in osteosarcoma. Mol. Cell. Biol.. 10: 5772–5781.
M.B. Kastan, O. Onyekwere, D. Sidransky, B. Vogelstein and R.W. Craig. 1991. Participation of p53 protein in the cellular response to DNA damage. Cancer Res.. 51: 6304–6311.
N. Stewart, G.G. Hicks, F. Paraskevas and M. Mowat. 1995. Evidence for a second cell cycle block at G2/M by p53. Oncogene. 10: 109–115.
R. Aloni-Grinstein, D. Schwartz and V. Rotter. 1995. Accumulation of wild-type p53 protein upon gamma irradiation induces a G2 arrest-dependent immunoglobulin kappa light chain gene expression. EMBO J.. 14: 1392–1401.
S.M. Cross, C.A. Sanchez, C.A. Morgan, M.K. Schimke, R. S., R. Idzerda, W.H. Raskind and B.J. Reid. 1995. A p53-dépendent mouse spindle checkpoint. Science. 267: 1353–1356.
S. Waga, G.J. Hannon, D. Beach and B. Stillman. 1994. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature. 369: 574–578.
Y. Luo, J. Hurwitz and J. Massague. 1995. Cell-cycle inhibition by independent CDK and PCNA binding domains in p2ICip1 Nature. 375: 159–161.
J. Chen, P. Jackson, M.W. Kirschner and A. Dutta. 1995. Separate domains of p21 involved in the inhibition of Cdk kinase and PCNA. Nature. 374: 386–388.
M.L. Smith, I.T. Chen, Q. Zhan, I. Bae, C.Y. Chen, T. Gilmer, M.B. Kastan, P.M. O’Connor and A.J.J. Fornace. 1994. Interaction of the p53-regulated protein Gadd45 with proliferating cell nuclear antigen. Science. 266: 1376–1380.
K. Okamoto and D. Beach. 1994. Gyclin G is a transcriptional target of the p53 tumor suppressor protein. EMBO J. 13: 4816–4822.
A. Zauberman, A. Lupo and M. Oren. 1995. Identification of p53 target genes through immune selection of genomic DNA: the cyclin G gene contains two distinct p53 binding sites. Oncogene. 10: (in press).
X.W. Wu, J.H. Bayle, D. Olson and A.J. Levine. 1993. The p53-mdm-2 autoregulatory feedback loop. Genes Dev. 7: 1126–1132.
Y. Barak, E. Gottlieb, T. Juven-Gershon and M. Oren. 1994. Regulation of mdm2 expression by p53: alternative promoters produce transcripts with nonidentical translation potential. Genes Dey. 8: 1739–1749.
Y. Barak, T. Juven, R. Haffner and M. Oren. 1993. mdm2 expression is induced by wild type-p53 activity. EMBO J. 12: 461–468.
M. Selvakumaran, H.K. Lin, T. Miyashita, H.G. Wang, S. Karajewski, J.C. Reed, B. Hoffman and D. Lieberman. 1994. Immediate early up-regulation of bax expression by p53 but not TGF beta I: a paradigm for distinct apoptotic pathways. Oncogene. 9: 1791–1798.
Q.M. Zhan, S.J. Fan, I. Bae, C. Guillouf, D.A. Liebermann, P.M. O’Connor and A.J. Fornace. 1994. Induction of bax by genotoxic stress in human cells correlates with normal p53 status and apoptosis. Oncogene. 9: 3743–3751.
T. Miyashita and J.C. Reed. 1995. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 80: 293–299.
Z.N. Oltvai, C.L. Milliman and S.J. Korsmeyer. 1993. bcl-2 heterodimerizes in vivo with a conserved homolog, bax, that accelerates programed cell death. Cell. 74: 609–619.
K.M. Dameron, O.V. Volpert, M.A. Tainsky and N. Bouck. 1994. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science. 265: 1582–1584.
D.P. Lane. 1992. p53, guardian of the genome. Nature. 358: 15–16.
M. Oren. 1994. Relationship of p53 to the control of apoptotic cell death. Seminars Cancer Biol. 5: 221–227.
E. Yonish-Rouach, D. Resnitzky, J. Lotem, L. Sachs, A. Kimchi and M. Oren. 1991. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by Interleukin-6. Nature. 352: 345–347.
E. Yonish-Rouach, D. Grunwald, S. Wilder, A. Kimchi, E. May, J.J. Lawrence, R May and M. Oren. 1993. p53-mediated cell death - relationship to cell cycle control. Mol Cell Biol. 13: 1415–1423.
N. Levy, E. Yonish-Rouach, M. Oren and A. Kimchi. 1993. Complementation by wild-type p53 of interleukin-6 effects on MI cells–induction of cell cycle exit and cooperativity with c-myc suppression. Mol. Cell. Bio l. 13: 7942–7952.
R. Shaw, R. Bovey, S. Tardy, R. Sahli, B. Sordat and J. Costa. 1992. Induction of apoptosis by wild-type p53 in a human colon tumor-derived cell line. Proc Natl Acad Sci USA. 89: 4495–4499.
T. Ramqvist, K.P. Magnusson, Y.S. Wang, L. Szekely, G. Klein and K.G. Wiman. 1993. Wild-type p53 induced apoptosis in a Burkitt lymphoma (BL) line that carries mutant p53. Oncogene. 8: 1495–1500.
Y.S. Wang, T. Ramqvist, L. Szekely, H. Axelson, G. Klein and K.G. Wiman. 1993. Reconstitution of wild-type p53 expression triggers apoptosis in a p53-negative v-myc retrovirus-induced T-cell lymphoma line. Cell Growth Differ. 4: 467–473.
J.J. Ryan, R. Danish, C.A. Gottlieb and M.F. Clarke. 1993. Cell cycle analysis of p53-induced cell death in murine erythroleukemia cells. Mol Cell Biol. 13: 711–719.
P. Johnson, S. Chung and S. Benchimol. 1993. Growth suppression of Friend virus-transformed erythroleukemia cells by p53 protein is accompanied by hemoglobin production and is sensitive to erythropoietin. Mol Cell Biol. 13: 1456–1463.
M. Debbas and E. White. 1993. Wild-Type p53 mediates apoptosis by E1A, which is inhibited by El B. Genes Dey 7: 546–554.
X.W. Wu and A.J. Levine. 1994. p53 and E2F-1 cooperate to mediate apoptosis. Proc Natl Acad Sci USA. 91: 3602–3606.
E. Yonish-Rouach, J. Borde, M. Gotteland, Z. Mishal, A. Viron and E. May. 1994. Induction of apoptosis by transiently transfected metabolically stable wtp53 in transformed cell lines. Cell Death Diff.. 1: 39–47.
S.W. Lowe, E.M. Schmitt, S.W. Smith, B.A. Osborne and T. Jacks. 1993. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 362: 847–849.
A.R. Clarke, C.A. Purdie, D.J. Harrison, R.G. Morris. C.C. Bird, M.L. Hooper and A.H. Wyllie. 1993. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 362: 849–852.
W. Maltzman and L. Czyzyk. 1984. UV-irradiation stimulates levels of p53 cellular tumor antigen in non-transformed mouse cells. Mol. Cell. Biol.. 4: 1689–1694.
S.W. Lowe, H.E. Ruley, T. Jacks and D.E. Housman. 1993. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 74: 957–967.
J. Lotem and L. Sachs. 1993. Hematopoietic cells from mice deficient in wild-type p53 are more resistant to induction of apoptosis by some agents. Blood. 82: 1092–1096.
E. Gottlieb, R. Haffner, T. Von Ruden, E.F. Wagner and M. Oren. 1994. Down-regulation of wild-type p53 activity interferes with apoptosis of IL-3-dependent hematopoietic cells following IL-3 withdrawal. EMBO J. 13: 1368–1374.
E. Gottlieb, R. Haffner, E. Yonish-Rouach, T. von Ruden, E. Wagner and M. Oren. 1994. Wild type p53 activity contributes to dependence on hematopoietic survival factors. Apoptosis (E. Mihich and R.T. Schimke, eds.). pp. 31–45. Plenum. New York.
Y.M. Zhu, D.A. Bradbury and N.H. Russell. 1994. Wild-type p53 is required for apoptosis induced by growth factor deprivation in factor-dependent leukaemic cells. Br J Cancer 69: 468–472.
C.E. Canman, T.M. Gilmer, S.B. Coutts and M.B. Kastan. 1995. Growth factor modulation of p53-mediated growth arrest versus apoptosis. Genes Dev.. 9: 600–611.
J. Martinez, I. Georgoff, J. Martinez and A.J. Levine. 1991. Cellular localization and cell cycle regulation by a temperature-sensitive p53-protein. Genes Del,. 5: 151–159.
J.V. Gannon and D.P. Lane. 1991. Protein synthesis required to anchor a mutant p53 protein which is temperature-sensitive for nuclear transport. Nature. 349: 802–806.
L. Sachs. 1990. The control of growth and differentiation in normal and leukemic blood cells. Cancer. 65: 2196–2206.
K. Vousden. 1993. Interactions of human papillomavirus transforming proteins with the products of tumor suppressor genes. FASEB J. 7: 872–879.
Y. Haupt, S. Rowan and M. Oren. 1995. p53-mediated apoptosis in HeLa cells can be overcome by excess pRB. Oncogene. 10: 1563–1571.
X.-Q. Qin, D.M. Livingston, W.J. Kaelin and P.D. Adams. 1994. Deregulated transcription factor E2F-1 expression leads to S-phase entry and p53-mediated apoptosis. Proc. Natl. Acad. Sci. USA. 91: 10918–10922.
E. Shaulian, I. Haviv, Y. Shaul and M. Oren. 1995. Transcriptional repression by the C-terminal domain of p53. Oncogene. 10: 671–680.
Y.J. Cho, S. Gorina, P.D. Jeffrey and N.P. Pavletich. 1994. Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations. Science. 265: 346–355.
J.Y. Lin, J.D. Chen, B. Elenbaas and A.J. Levine. 1994. Several hydrophobic amino acids in the p53 amino-terminal domain are required for transcriptional activation, binding to mdm-2 and the adenovirus 5 E1B 55-kD protein. Genes Dev. 8: 1235–1246.
D. Michael-Michalovitz, F. Yehiely, E. Gottlieb and M. Oren. 1991. Simian virus-40 can overcome the antiproliferative effect of wild-type-p53 in the absence of stable large t-antigen-p53 binding. J. Virol. 65: 4160–4168.
R.S. Quartin, C.N. Cole, J.M. Pipas and A.J. Levine. 1994. The amino-terminal functions of the simian virus 40 large T antigen are required to overcome wild-type p53-mediated growth arrest of cells. J Virol. 68: 1334–1341.
R.J.C. Slebos, M.H. Lee, B.S. Plunkett, T.D. Kessis, B.O. Williams, T. Jacks, L. Hedrick, M.B. Kastan and K.R. Cho. 1994. p53-dependent GI arrest involves pRB-related proteins and is disrupted by the human papillomavirus 16 E7 oncoprotein. Proc Natl Acad Sci USA. 91: 5320–5324.
H. Pan and A.E. Griep. 1994. Altered cell cycle regulation in the lens of HPV-16 E6 or E7 transgenic mice: implications for umor suppressor gene funciton in development. Genes Dev. 8: 1285–1299.
A.E. White, E.M. Livanos and T.D. Tlsty. 1994. Differential disruption of genomic integrity and cell cycle regulation in normal human fibroblasts by the HPV oncoproteins. Genes Dev. 8: 666–677.
G.W. Demers, S.A. Foster, C.L. Halbert and D.A. Galloway. 1994. Growth arrest by induction of p53 in DNA damaged keratinocytes is bypassed by human papillomavirus 16 E7. Proc Natl Acad Sci USA. 91: 4382–4386.
E.S. Hickman, S.M. Picksley and K.H. Vousden. 1994. Cells expressing HPV 16 E7 continue cell cycle progression following DNA damage induced p53 activation. Oncogene. 9: 2177–2181.
S.D. Morgenbesser, B.O. Williams, T. Jacks and R.A. Depinho. 1994. p53-dependent apoptosis produced by Rb-deficiency in the developing mouse lens. Nature. 371: 72–74.
M.L. Smith, Q.M. Zhan, I.S. Bae and A.J. Fornace. 1994. Role of retinoblastoma gene product in p53-mediated DNA damage response. Exp Cell Res. 215: 386–389.
S.W. Lowe and H.E. Ruley. 1993. Stabilization of the p53 tumor suppressor is induced by adenovirus-5 El A and accompanies apoptosis. Genes Dev. 7: 535.
H. Hermeking and D. Eick. 1994. Mediation of c-myc-induced apoptosis by p53. Science. 265: 2091–2093.
A.J. Wagner, J.M. Kokontis and N. Hay. 1994. Myc-mediated apoptosis requires wild-type p53 in a manner independent of cell cycle arrest and the ability of p53 to induce p21waf1/cip1. Genes Dev. 8: 2817–2830.
C. Caelles, A. Helmberg and M. Karin. 1994. p53-dependent apoptosis in the absence of transcriptional activation of p53 target genes. Nature. 370: 220–223.
T. Crook, N.J. Marston, E.A. Sara and K.H. Vousden. 1994. Transcriptional activation by p53 correlates with growth suppression but does not preclude the acquisition of transforming function. Cell. 79: 817–827.
K. Ory, Y. Legros, C. Auguin and T. Soussi. 1994. Analysis of the most representative tumour-derived p53 mutants reveals that changes in protein conformation are not correlated with loss of transactivation or inhibition of cell proliferation. EMBO J.. 13: 3496–3504.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1996 Springer Science+Business Media New York
About this chapter
Cite this chapter
Haupt, Y. et al. (1996). P53-Mediated Apoptosis. In: Mihich, E., Housman, D. (eds) Cancer Genes. Pezcoller Foundation Symposia, vol 7. Springer, Boston, MA. https://doi.org/10.1007/978-1-4615-5895-8_5
Download citation
DOI: https://doi.org/10.1007/978-1-4615-5895-8_5
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4613-7704-7
Online ISBN: 978-1-4615-5895-8
eBook Packages: Springer Book Archive