Abstract
Angiosperm double fertilization is composed of two sets of gamete fusion, in which two sperm cells released from a pollen tube fuse with egg and central cells to produce an embryo and an endosperm, respectively. GCS1 was, for the first time, identified as a sperm membrane protein essential for plant gamete fusion, and this indicates that plant fertilization is regulated by molecular systems on the gamete surface. Interestingly, GCS1 is conserved in various phylogenetically distant organisms, such as algae, unicellular parasites, slime molds, and some invertebrate species, and Plasmodium and Chlamydomonas GCS1 orthologues have proved to similarly function in their gamete fusion as a male factor. This finding indicates that GCS1 is an ancient fertilization regulator; thus, elucidating GCS1-based gamete fusion mechanism should lead to understanding of conserved eukaryotic fertilization system. Because GCS1 is a novel transmembrane protein in which no well-known functional domains are present, the molecular mechanics of GCS1-based fertilization remain elusive.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
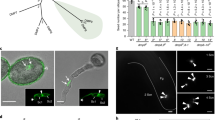
In a previous study using male gametic cells (generative cells) isolated from the trumpet lily (Lilium longiflorum), GENERATIVE CELL SPECIFIC 1 (GCS1 ), also called HAP2, was identified as a critical factor for flowering plant gamete fusion (Mori et al. 2006). GCS1 is a novel transmembrane protein composed of approximately 700–1,000 amino-acid residues, and it possesses a single transmembrane domain (Mori et al. 2006). In flowering plants (L. longiflorum and Arabidopsis thaliana), functional GCS1 expression is specific to male gametes and GCS1 protein is detected exclusively in male gametes (generative cells and sperm cells) suspended in a pollen grain (Mori et al. 2006; von Besser et al. 2006). The typical fertilization of flowering plants involves two sets of gamete fusion and is performed by a pair of sperm cells delivered by pollen tube elongation. When the sperm cells are released into an embryo sac enclosed in an ovule, one of them fuses with an egg cell, and the other fuses with a central cell to produce an embryo and an endosperm, respectively (Fig. 26.1). This fertilization type, which is specific to angiosperm species, is known as “double fertilization .” Reverse-genetic approaches using Arabidopsis GCS1 mutants clearly demonstrated that no GCS1-knockout sperm cells are able to fuse with female gametes (egg and central cells) (Mori et al. 2006). This finding became the first with respect to the plant gamete fusion factor. Interestingly, GCS1 orthologues have been detected in various organisms from unicellular protists (e.g., green and red algae, slime molds, unicellular parasites) to some animal species (e.g., arthropods, cnidarians) in addition to plants (Mori et al. 2006; von Besser et al. 2006; Liu et al. 2008; Hirai et al. 2008; Steele and Dana 2009). Of the GCS1-possessing protists, the malaria parasite (Plasmodium berghei) and Chlamydomonas reinhardtii show functional GCS1 expression specific to a particular sex gamete corresponding to the male, similar to flowering plants (Liu et al. 2008; Hirai et al. 2008). Furthermore, GCS1-knockout lines of those organisms show serious sterility, resulting in unsuccessful zygote formation (Liu et al. 2008; Hirai et al. 2008). Because the GCS1-knockout male gametes in both Plasmodium and Chlamydomonas succeed in gamete attachment, the GCS1 molecule may function in gamete membrane fusion itself or in processes close to the membrane fusion step following gamete attachment. In addition, a recent study has begun to analyze animal GCS1 function and showed that Hydra magnipapillata GCS1 is specifically expressed in its testis (Steele and Dana 2009). These findings strongly suggest that GCS1 is a highly conserved gamete fusion factor and characterizes male fertility in plants, animals, and protists.
2 GCS1 Functions at the Gamete Attachment Site
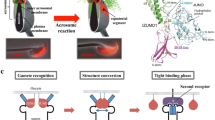
The expression pattern of GCS1 appears to vary by species. In Chlamydomonas, GCS1 expression is exclusive to the mt − (male)-side mating structure (mating protrusion), in which gamete membrane fusion occurs, and this is consistent with the fact that mt + (female)-specific gamete fusion factor FUS1 is expressed specifically in mt +-side mating structures (Liu et al. 2008) (Fig. 26.2). In contrast to Chlamydomonas, GCS1 molecules are uniformly distributed in male gametes of plants and the malaria parasite (Mori et al. 2006; von Besser et al. 2006; Liu et al. 2008; Hirai et al. 2008). The Plasmodium male gamete enters the female gamete until initiating fusion (Sinden and Croll 1975), and therefore gamete fusion via GCS1 function may occur on the entire male gamete surface after the male gamete is engulfed by the female gamete (Fig. 26.2). Interestingly, a recent study showed detailed changes in the distribution of Arabidopsis GCS1 in sperm cells (Sprunck et al. 2012). Some studies showed that Arabidopsis GCS1 is mainly detected in the internal membrane components surrounding the sperm nucleus, although slight GCS1 localization is also observed on the sperm surface (von Besser et al. 2006; Igawa et al. 2013). Sprunck et al. (2012) identified a novel egg cell-specific secretory protein, named EGG CELL 1 (EC1 ), and showed that EC1 family peptides secreted into the gamete fusion space of the embryo sac induce a shift of GCS1 distribution toward the sperm surface membrane, likely to ensure that the GCS1 molecules directly contact the female gametes (Fig. 26.2). As EC1 is specific to plant species, this EC1-based sperm–egg crosstalk system may have been developed for correct double-fertilization progression. At the same time, this finding strongly supports GCS1 as a vital player for gamete fusion at the membrane attachment site in plant species.
GENERATIVE CELL SPECIFIC 1 (GCS1) -possessing organisms and their gamete fusion method based on GCS1. In C. reinhardtii, GCS1 is localized specifically on the male-side mating structure, and the GCS1 molecules face the female-side mating structure during fertilization. In Plasmodium (P.) berghei, GCS1 is uniformly distributed on the male gamete surface, and the male gamete enters the female gamete during fertilization. In A. thaliana, the GCS1 molecules are mainly kept in the sperm cytoplasm. When the sperm cells arrive at an egg cell, they are activated by EC1 molecules secreted from the egg cell. The activated sperm cells transfer GCS1 toward the cell surface
3 Characterization of the GCS1 Molecule
Since the identification of GCS1 , researchers have tried to elucidate the functional mechanism of GCS1 because it is a novel protein with no characterized domains (Mori et al. 2006). Recently, a pair of reports on Arabidopsis GCS1 structures were released (Wong et al. 2010; Mori et al. 2010). The general GCS1 structure is composed of an N-terminal signal sequence, a highly conserved domain (HAP2-GCS1 domain), a transmembrane (TM) domain, and a positively charged C-terminal domain (Fig. 26.3a) (Wong et al. 2010; Mori et al. 2010). Wong et al. (2010) produced various Arabidopsis GCS1 constructs, in which the entire N-terminal (AtN) sequence before the TM domain or the entire C-terminal (AtC) sequence after the TM domain was deleted, and introduced these constructs into an Arabidopsis GCS1 mutant to determine whether such truncated GCS1s are functional. As a result, both the N- and C-terminus-deleted GCS1s were shown to be nonfunctional. In addition, they showed that an N-terminal GCS1 sequence derived from Sisymbrium irio, which is phylogenetically near A. thaliana, complemented the Arabidopsis GCS1 mutation but that of Oryza sativa GCS1 did not. However, the Oryza GCS1 C-terminus did not affect the Arabidopsis GCS1 function. Furthermore, they produced Arabidopsis GCS1 constructs in which the positively charged C-terminal domain was modified and showed that these positively charged amino-acid residues are also important for GCS1 function. However, Mori et al. (2010) reported that the majority of the GCS1 C-terminus is dispensable for GCS1 function. In their paper, various modified Arabidopsis GCS1 constructs, in which each of the characteristic domains is disrupted by a GFP cDNA insertion, were produced and similarly introduced into Arabidopsis GCS1 mutants. As a result, although most of the C-terminal sequence was exchanged for the GFP (green fluorescent protein) sequence, the modified GCS1 retained its gamete fusion function at a level comparable with that of normal GCS1. In the same paper, a similar result was also obtained in the case of modified P. berghei GCS1, in which the entire C-terminal sequence was deleted. Although the detailed cause of the conflict between the findings of these two groups remains unclear, the contribution of the C-terminus to gamete fusion function may not be generalized among all GCS1-possessing organisms.
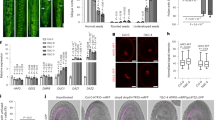
Expression of secretory-type GCS1 (GAH) and gamete fusion inhibition. (a) Arabidopsis GCS1 is composed of an N-terminal signal sequence (SS), a highly conserved domain (HAP2-GCS1), a transmembrane domain (TM), and a positively charged histidine-rich domain (HR). In the GAH protein, the TM domain has been exchanged for a GFP sequence. (b) GAH was expressed specifically in an egg cell by the DD45 promoter. The GAH molecules were expected to be secreted from the egg cell and then to block the putative GCS1 receptor on the egg surface. (c–e) Observation of GAH expression. The egg cell (EC) detected by differential interference contrast microscopy (c) showed both egg nucleus (EN) RFP (d) and GFP signals (e) from the GAH molecules. (f) In the GAH-expressing lines, unfused sperm cells were frequently detected (arrows). Insert is a magnification of the region indicated by the top arrow and shows a pair of RFP signals from unfused sperm cell nuclei (SN). Bars c 20 μm; f 50 μm
4 A New Attempt to Elucidate the Mechanism of GCS1-Based Gamete Fusion
The N-terminal GCS1 sequence is expected to be exposed on the male gamete surface as an extracellular domain because of the presence of the N-terminal signal sequence and C-terminal single-pass transmembrane domain. These features also suggest that the C-terminus following the TM domain should be a cytosolic domain. Based on this expectation and the aforementioned findings of the Mori group, GCS1 may function as a type of ligand attached on the male gamete surface, because if it is a receptor-like molecule, the cytosolic C-terminus should function in internal signal transduction similar to a receptor kinase. However, as shown by the Mori group, the C-terminus is dispensable for GCS1 function, suggesting that only the extracellular N-terminus is functional in gamete fusion. In addition, if GCS1 functions as a ligand, the female gamete may express a partner molecule functioning as a GCS1 receptor to produce a gamete fusion complex on the surface. Recently, a modified Arabidopsis GCS1 gene construct, in which the TM domain was exchanged for GFP cDNA under an egg cell-specific promoter (pDD45), was newly produced and named pDD45::GAH (unpublished data) (Fig. 26.3a). Because the GAH peptide does not possess the TM domain, the GAH peptide produced in the egg cell was expected to be secreted into the gamete fusion space of the embryo sac and to prevent sperm–egg fusion by blocking the GCS1 partner (Fig. 26.3b). As a result, when the pDD45::GAH construct was introduced into an Arabidopsis egg nucleus marker line expressing pEC1 ::H2B-RFP, the GFP signal derived from the GAH peptide was detected specifically in the egg cell (unpublished data) (Fig. 26.3c–e). Next, the GAH-expressing line was pollinated with pollen from a sperm nucleus marker line expressing pHTR10::HTR10-RFP to determine whether gamete fusion was prevented. Unfused sperm cells were occasionally detected in the ovules (unpublished data) (Fig. 26.3f). Notably, both sperm cells were prevented from fusing, suggesting that the GAH peptide blocked not only the sperm–egg fusion but also the sperm–central cell fusion. These observations also support GAH secretion. Based on these results, GCS1 molecules are expected to bind female counterpart(s) most likely expressed on both the egg and central cells. The GAH-expressing line may be useful to isolate such GCS1-partner molecules in the future.
5 Perspectives
Even before the identification of GCS1 , some transmembrane proteins critical to gamete fusion had been identified and characterized in some species, such as Chlamydomonas FUS1 , Caenorhabditis elegans Spe-9 , and mammalian IZUMO1 (Twell 2006). Because of species specificity, those proteins may have been developed to facilitate species-specific gamete fusion processes. However, given that the final process of gamete fusion is based upon lipid bilayer membrane contact in any species, there must be gamete fusion processes shared by different sexual reproductive organisms. GCS1 is a promising protein to help elucidate such conserved gamete fusion systems, and any approaches used to study GCS1 should lead to a fundamental understanding of these systems.
References
Hirai M, Arai M, Mori T et al (2008) Male fertility of malaria parasites is determined by GCS1, a plant-type reproduction factor. Curr Biol 18:607–613
Igawa T, Yanagawa Y, Miyagishima SY et al (2013) Analysis of gamete membrane dynamics during double fertilization of Arabidopsis. J Plant Res 126:387–394
Liu Y, Tewari R, Ning J et al (2008) The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes Dev 22:1051–1068
Mori T, Kuroiwa H, Higashiyama T et al (2006) GENERATIVE CELL SPECIFIC 1 is essential for angiosperm fertilization. Nat Cell Biol 8:64–71
Mori T, Hirai M, Kuroiwa T et al (2010) The functional domain of GCS1-based gamete fusion resides in the amino terminus in plant and parasite species. PLoS One 5:e15957
Sinden RE, Croll NA (1975) Cytology and kinetics of microgametogenesis and fertilization in Plasmodium yoelii nigeriensis. Parasitology 70:53–65
Sprunck S, Rademacher S, Vogler F et al (2012) Egg cell-secreted EC1 triggers sperm cell activation during double fertilization. Science 338:1093–1097
Steele RE, Dana CE (2009) Evolutionary history of the HAP2/GCS1 gene and sexual reproduction in metazoans. PLoS One 4:e7680
Twell D (2006) A blossoming romance: gamete interactions in flowering plants. Nat Cell Biol 8:14–16
von Besser K, Frank AC, Johnson MA et al (2006) Arabidopsis HAP2 (GCS1) is a sperm-specific gene required for pollen tube guidance and fertilization. Development (Camb) 133:4761–4769
Wong JL, Leydon AR, Johnson MA (2010) HAP2(GCS1)-dependent gamete fusion requires a positively charged carboxy-terminal domain. PLoS Genet 6:e1000882
Acknowledgments
This study is funded by a Grant-in-Aid for Scientific Research on Innovative Areas (21112008) and a Grant-in-Aid for Young Scientists (B) (24770062), to T. M., from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
This chapter is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
Copyright information
© 2014 The Author(s)
About this paper
Cite this paper
Mori, T. (2014). Profiling the GCS1-Based Gamete Fusion Mechanism. In: Sawada, H., Inoue, N., Iwano, M. (eds) Sexual Reproduction in Animals and Plants. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54589-7_26
Download citation
DOI: https://doi.org/10.1007/978-4-431-54589-7_26
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54588-0
Online ISBN: 978-4-431-54589-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)