Abstract
Eight food-deprived Wistar rats developed stable patterns of lever pressing and licking when exposed to a fixed-time 30-s schedule of food pellet presentation. The rats were trained to lever press by presenting the lever 10 s before the programmed food delivery, with the food pellet being delivered immediately upon a lever press. The operant contingency was then removed and the lever was inserted through the entire interfood interval, being withdrawn with food delivery and reinserted 2 s later. On successive phases of the study, a protective contingency postponed food delivery if responses (lever presses or licks) occurred within the last 1, 2, 5, 10, 20, or 25 s of the interfood interval. Lever pressing was reduced at much shorter response–food delays than those that reduced licking. These results demonstrate that reinforcement contributes to the maintenance of different response patterns on periodic schedules, and that different responses are differentially sensitive to delays.
Similar content being viewed by others
Schedule-induced behavior is behavior that occurs in excess when reinforcers are programmed intermittently, when there is no explicit contingency arranged between the occurrence of the behavior and the delivery of the reinforcer (for reviews, see Reid & Staddon, 1990; Wetherington, 1982). The first published experimental demonstration of schedule-induced behavior was by Falk (1961), who found that food-deprived but not water-deprived rats consumed unusual and excessive amounts of water concurrently with their execution of operant lever pressing that was intermittently reinforced by food. Falk (1961) termed this behavior “schedule-induced polydipsia,” because the drinking was excessive and there was no apparent contingency between the behavior and the delivery of food. Furthermore, Falk (1971) argued that schedule-induced polydipsia was the prototype of a category of behavior that he termed “adjunctive,” differentiating it from operant behavior on the basis of being induced rather than controlled by the reinforcement schedule. Clark (1962) disagreed, suggesting that schedule-induced polydipsia results from adventitious reinforcement of licking by food delivery.
Staddon (1977) extended and integrated the analysis of adjunctive and operant behavior by suggesting that schedule-induced behavior could be divided into interim and terminal activities. Such a classification depended on the nature of the behavior and on its temporal location within interfood intervals, with adjunctive behavior being equivalent to interim activity and operant behavior being instances of terminal activity (see also Staddon & Simmelhag, 1971). Interim activities occur at the beginning of the interfood interval when reinforcement probability is low, and terminal activities occur at the end of the interfood interval when reinforcement probability is high, thus laying the groundwork for a Pavlovian account of schedule-induced behavior in which the two forms are based on inhibitory and excitatory conditioned states, respectively (Lashley & Rosellini, 1980).
Differences in the sensitivity to delays of reinforcement depend on whether the behavior is interim or terminal (i.e., operant; cf., e.g., Flory & Lickfett, 1974, on adjunctive drinking, and Dickinson, Watt, & Griffiths, 1992, on operant lever pressing). Interim activities such as schedule-induced polydipsia were initially shown to be quite insensitive to response-dependent food delays (see, however, Pellón & Blackman, 1987, 1991), thus grounding the thesis of Falk (1971) that adjunctive is an entirely different category of behavior than operant. Falk (1964) himself reported that the amount of schedule-induced polydipsia in two rats exposed to a variable-interval 1-min schedule of food reinforcement was not eliminated, or even reduced, by the imposition of a contingency that ensured a delay in food delivery of at least 15 s from the last lick. Similarly, Hawkins, Schrot, Githens, and Everett (1972) reported that well-established drinking induced by a fixed-time (FT) 1-min schedule of food delivery (in which no operant response was required for food to be delivered) was not reduced by lick-dependent delays as long as 4 or 5 min.
Flory and Lickfett (1974) reported that schedule-induced drinking was relatively resistant to the effects of lick-dependent delays in reinforcement, contrasting with studies on operant lever pressing (cf. Dickinson et al., 1992). Flory and Lickfett found that rats’ schedule-induced drinking was never eliminated, although it was systematically reduced as the duration of lick-contingent time-outs from a fixed-interval schedule (FI 1 min) was increased through four values, from 10 to 80 s. During these periods, the operant response lever was retracted from the experimental chamber, and the timer that controlled the FI schedule was stopped. With 80-s periods of such lever withdrawal, drinking was consistently reduced, and with 40-s and 20-s periods, it was reduced in some animals. Similar resistance to the effects of lick-dependent delays in food delivery has been reported on the acquisition of schedule-induced drinking (Falk, 1964; Hawkins et al., 1972; Moran & Rudolph, 1980; Segal & Oden, 1969).
More recent studies have shown that schedule-induced drinking can be substantially reduced by short (10 s or less) lick-contingent delays in food delivery, provided that every lick effectively initiated a delay in food delivery and that the food delays were signaled by an external event. Such a result has been demonstrated repeatedly (e.g., Lamas & Pellón, 1995b; Pellón & Blackman, 1987), even when delays were unsignaled, and is in line with what should be expected from a standard operant response. Development of schedule-induced drinking can also be attenuated by short lick-dependent delays in food presentation (e.g., Lamas & Pellón, 1995a, 1997; Pellón & Blackman, 1991). In all cases, procedures were incorporated to separate the effect of the contingent delays from reductions in behavior potentially produced by the decreases in food frequency that inevitably accompanied the increases in the interfood interval length. A typical tactic was to utilize a yoked control.
Despite the above comparisons between adjunctive and operant behaviors, no single study has systematically compared the efficacy of response–food delays for reducing interim and terminal activities simultaneously. In the present study, rats’ licking and lever pressing were first established by presenting food at regular times independently of behavior; then, delays of increasing length were introduced between the last response of either type and food delivery, in order to measure the relative resistances of licking and lever pressing to reduction by response-dependent food delays. The manners in which response–food delays affect lever pressing and schedule-induced licking will clarify the nature of the effects of delays on adjunctive behavior, but they will also contribute to our understanding of the maintenance of such behavior by reinforcement. The introduction of delays of reinforcement contingent upon responding has the result of weakening the response–reinforcer relation, and thus behavior should decrease if its occurrence depends on strengthening by reinforcement (see Lattal, 2010). Because operant responses can be acquired and maintained with long delays between the behavior and the reinforcer (e.g., D’Amato, Safarjan, & Salmon, 1981; Lattal & Gleeson, 1990), adjunctive behavior may be acquired and maintained in the same way (Killeen & Pellón, in press; see also Pellón, 2004).
Method
Subjects
Eight experimentally naïve male Wistar rats obtained from Charles River Laboratories (Lyon, France) were used. They were housed individually in a room with controlled environmental conditions (ambient temperature 21 °C, 60 % relative humidity, and an 8:00 am/8:00 pm light/dark cycle). The rats’ weights were gradually reduced by controlled feeding to 80 % of their free-feeding body weights and were maintained at this percentage with reference to their ideal growth curve (provided by Charles River). At the beginning of the study, the animals were approximately 65 days old and had a mean body weight of 304.50 g (range: 293–323 g). Rats were weighed daily and were given the supplement of food necessary to maintain their required weights. Water was freely available to all animals in their home cages. All animal care procedures were in accordance with the European Union Council Directive 2010/63 and the Spanish Royal Decree 1201/2005 for minimizing stress and discomfort in animals.
Apparatus
The study was conducted in eight 29 × 24.5 × 35.5 cm Letica Instruments LI 836 two-lever rodent conditioning chambers (Barcelona, Spain). The chambers were enclosed in soundproofed housing equipped with a ventilation system and a small observation window in the left panel. The front panel of each chamber was made of aluminum, the left-hand wall and roof of transparent Plexiglas, and the remaining sides of black Plexiglas. The right lever of each chamber was permanently withdrawn during the experiment. A water bottle was attached to the external side of the right wall of each chamber, with its spout being accessible to the rat through a 3.2 × 3.9 cm aperture, located 20 cm from the front wall and 7 cm above the floor. The spout was positioned 2 cm from the wall aperture, in such a way that the rat could lick but not maintain permanent contact with it. Licks at the spout were detected when the electric circuit between the 16 parallel metal bars comprising the grid floor and the drinking bottle spout was completed via contact with the animal’s tongue (which did not produce any shock to the animal). The chambers were illuminated by two internal 3-W bulbs, placed on the upper part of the front panel to either side of the food tray, and a 25-W ambient light fitted to the external housing. The ambient noise produced by the ventilation fan was 60 dB, which served to mask any other external sounds. A Letica Instruments dispenser fitted to the outside of the front panel could deliver 45-mg food pellets (Bio-Serv) into a food tray, situated in the center of the front wall at a height of 3.7 cm above the floor. Programming and recording of events were performed with the MED-PC software for Windows, version 1.15.
Procedure
When the animals’ weights were stabilized at 80 % of the original free-feeding weights, they were exposed to a FT 30-s food delivery schedule by which a single food pellet was presented at regular 30-s intervals, regardless of the animals’ behavior. During the first phase of the study, water access and free opportunities to press the left-side lever were available during the entire interfood interval (Stage 1:1). This lasted for eight sessions, each 30 min in duration. Because the animals developed high rates of schedule-induced licking but low rates of schedule-induced lever pressing, in the second phase of the study they were exposed for four sessions to the same FT schedule with continuous access to water but with access to the lever just during the last 15 s of each interfood interval (Stage 1:0.5). In the third phase, which lasted for six sessions of 20 min each, the food schedule was changed to FT 40-s and lever pressing was restricted to the last 10 s of each interfood interval, while water was available throughout the whole interfood interval (Stage 1:0.25). In the fourth phase (five sessions), the levers were still available only during the last quarter of the interfood intervals, but in addition the first lever press cancelled the interval, resulting in immediate delivery of the food pellet (an operant contingency; Stage 1:≤0.25). This was done in order to help initiate lever pressing. Given that animals showed significant increases in lever pressing, the operant contingency was removed, and the conditions of Stage 1:0.25 were reinstated for an additional ten sessions. The original conditions were reestablished under the FT 30-s food schedule for ten final sessions of 15-min length (Stage 1:1), with the slight modification that, while water was continuously available, the lever was retracted immediately before the delivery of each food pellet and reinserted 2 s later, thus being present for only 28 s of each 30-s interfood interval. In order to maintain lever pressing, we followed the procedure of previous autoshaping research, in that the lever was retracted at the time of food delivery, thus eliciting contact with it when the lever was reinserted into the chamber (Davey & Cleland, 1982; Locurto, Terrace, & Gibbon, 1976).
As a result of the training history detailed above, animals licked and pressed readily in circumstances in which food pellets were presented according to a FT 30-s schedule. On successive phases of the study, a protective contingency postponed food delivery if responses (lever presses or licks) occurred within the last 1, 2, 5, 10, 20, 25, or 30 s of the interfood intervals (i.e., DRO—“differential reinforcement of other behavior”—contingencies). During these phases, therefore, the food schedule could be described as tandem FT DRO, with each phase lasting ten sessions. Every session of each experimental phase terminated after 30 food pellets were delivered. As a final experimental phase, delays were discontinued and recovery of the initial FT 30-s schedule was implemented for ten sessions.
For each session, the number of licks at the water spout, the number of lever presses, the number of food pellets delivered, and the total duration of the session were recorded for each rat. Licks and lever presses were also recorded for every 1-s bin within the interfood intervals. Statistical analyses were conducted with SPSS version 17.0.
Results
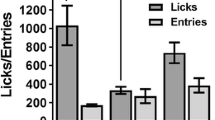
Schedule-induced drinking was rapidly acquired when animals were exposed to a schedule on which a single pellet of food was delivered intermittently and independently of any behavior (an FT 30-s schedule), but schedule-induced lever pressing was not. Such differential acquisition can be seen at the left of Fig. 1, under the 1:1 heading during the first phase of the study (indicating that both the water bottle and the lever were present throughout the whole interfood interval). When the lever was available only during the second half of the interfood intervals during the second phase of the study (1:0.5), the level of schedule-induced licking was maintained, but lever pressing again did not develop. In the third phase, the lever was inserted only during the last quarter of the interfood interval (1:0.25), which was increased to 40 s. Under these conditions, which also involved a reduction in session length and the number of food pellets delivered (from 60 to 30), total licking decreased to about half the former level and initial signs of lever pressing emerged. To further increase lever pressing, a contingency was added so that the first lever press of each interfood interval was followed immediately by food delivery and the cancellation of the interval (Stage 1:≤0.25), resulting in an effective increase in lever pressing and the maintenance of schedule-induced licking. The subsequent removal of the operant contingency (Stage 1:0.25) and the final extension of the lever to occupy almost the entire interfood interval (Stage 1:1) maintained significant levels of licking and lever pressing until the end of training.
Means (± standard errors) of total licks and lever presses for each session of the different acquisition phases of the experiment, where animals had constant access to a water bottle throughout, but limited access to the lever during specific experimental stages (see the text for a detailed explanation)
After rats developed stable patterns of lever pressing and schedule-induced licking, protective response–food delays of different durations were introduced in successive phases. Figure 2 shows the total numbers of licks and lever presses for each delay relative to baseline, averaged across the last three sessions of each delay value. Response totals are expressed as percentages of the baseline value because these baselines differed across phases (see Fig. 1).
Percentages of change with respect to the final baseline level of acquisition of licking and lever pressing for conditions in which protective food delays were imposed on responding occurring during the last 1, 2, 5, 10, 20, 25, or 30 s of the interfood intervals. Each point represents the mean and standard error for all rats during the last three sessions at each delay value
As Fig. 2 shows, the DRO contingency arranged for the last 1, 2, 5, 10, 20, 25, or 30 s of interfood intervals led to progressive reductions in both lever pressing and licking as the delay value increased. Lever pressing was sensitive to response–food delays that were much shorter than those that reduced licking, showing a sharp decline in responding with short response–food delays. The percentage reductions of total lever presses with delays of 1 and 2 s were approximately 62 % and 84 %, respectively. With further delays, lever pressing continued to decline, especially at 25- or 30-s delays. The percentage reduction of total licking decreased monotonically as delay values were increased, and only showed decreases below 50 % at the very long 25- and 30-s lick–food delays. Delays of 1 s produced decreases of about 40 %, but then 2-s delays were completely ineffective in reducing licking (showing even small increases), and food delays of up to 20 s were required to reach again a 40 % reduction.
To substantiate these observations, a 2 × 8 within-subjects ANOVA was performed with Response Type (licks and lever presses) and Delay (the different delay values, including the 0-s delay condition) as factors. The ANOVA on total responses confirmed that the effects of both response [F(1, 14) = 11.70, p = .004] and delay [F(7, 98) = 5.83, p < .001] were statistically significant, as was their interaction [F(7, 98) = 3.83, p = .001]. This result supports the view that the delay had differential effects on licking and lever pressing. Whereas reductions in lever pressing occurred at very short delays, licking was only significantly reduced when delays were 10 s or higher (p < .05, as shown by Newman–Keuls tests; in addition to the significant reduction in licking at the 1-s delay).
The final recovery phase (Fig. 2, right-hand panel) showed that schedule-induced licking clearly increased up to about 80 % of baseline when delays were discontinued. In contrast, only a relatively low level of lever pressing (about 5 % of baseline) was observed at the end of the experiment.
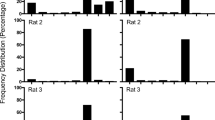
Figure 3 shows the responding for individual rats during the last three sessions of each delay value as percentages of the final baseline level. For most rats, the patterns of responding were similar to those for the average data (Fig. 2), namely that lever pressing decreased more rapidly than licking as a function of increasing response–food delays. The exceptions were Rat 3, which showed no difference between licking and lever pressing, and Rat 4, which showed the reverse pattern, with licking being more sensitive than lever pressing to food delays.
Individual data pertaining to percentages of change with respect to the final baseline level of acquisition of licking and lever pressing for conditions in which protective food delays were imposed on responding occurring during the last 1, 2, 5, 10, 20, 25, or 30 s of the interfood intervals. Each point represents the mean and standard error of the last three sessions at each delay value
Figure 4 shows the rates of food delivery throughout the experiment, at each delay value. Single data points correspond to individual subjects, and the solid line represents the average. As can be seen, food frequency did not change much with the introduction of delays of increasing length, although small decreases did occur at delay values of 20, 25, and 30 s. Session duration did not change much with the introduction of response–food delays, because the delays were quite effective at reducing responding as they made contact with lever pressing or licking (see the data in Fig. 5). This was even true for the first sessions as different delays were introduced. The maximum mean session duration was 16.30 min for the first session under the 25-s delay condition.
Figure 5 shows the temporal distributions of schedule-induced licking (in black) and lever pressing (in white) for the baseline and recovery phases (both with 0-s delays) and for each of the delay conditions of the study, each represented in a separate panel. For each panel, total licks and total presses are shown for 1-s bins throughout the whole interfood interval, averaged over the last three sessions of each phase. Under a simple FT 30-s food schedule (without the DRO contingency) schedule-induced licking showed the typical inverted U-shaped function relating licks to the interfood interval, with maximum licking in the first half of the interval and a peak at 10 s (see the uppermost left panel). Lever pressing showed a peak of responding at 3 s of the interpellet interval, then a decline followed by a steady increase, and finally sustained responding during the second half of the interpellet interval.
When protective delays were imposed between the last response (either a lick or a lever press) and food delivery, the temporal distributions of responding changed accordingly. The licking distribution was maintained well up to the 10-s delay, but the lever pressing distribution flattened with delays of 2 s or longer. Licking peaked around 10 s for delay values from 1 to 10 s. With lick–food delays of 20 s, the distribution of responding flattened, and this sustained, relatively low-rate responding was also maintained at 25- or 30-s delays, albeit at a lower level. For lever pressing, the 1-s delay produced response distributions similar to baseline (i.e., a peak at 3 s followed by a decline and a subsequent increase in responding as the interfood interval progressed, and sustained at a low level during the second half of the interval), but with delays of 2 s or longer, lever pressing simply disappeared. When delays were discontinued during the last recovery phase (lower rightmost panel) licking resumed, but not lever pressing.
Discussion
Lever pressing was maintained by a FT food delivery schedule, as was schedule-induced licking. Animals licked the bottle spout after each food delivery, thus conforming to a pattern typical of schedule-induced polydipsia (Falk, 1961; Flores & Pellón, 1995). The maintenance of lever pressing (albeit aided by some sessions of explicit positive reinforcement) was a novel finding of the present study. This finding is in line with previous demonstrations of autoshaping of lever pressing in rats (e.g., Davey & Cleland, 1982; Locurto et al., 1976) and supports Staddon’s (1977) suggestion that rats’ operant lever pressing is facilitated by its link to schedule-induced terminal behavior. Locurto et al. found that autoshaping resulted in more lever contacts than did omission training or random control procedures. Autoshaping trials were presented on average every 90 s (a variable-time 90-s intertrial interval) and consisted of the insertion of the lever (the conditioned stimulus) for 15 s and the delivery of a single pellet of food (the unconditioned stimulus) upon its withdrawal. Davey and Cleland also reported autoshaping of lever contact in rats; here, the lever insertions lasted 10 s and were presented on average every 100 s. The novel aspect of the present results is that pressing occurred to a lever that extended for 28 s, peaking as soon as the lever was inserted into the chamber (an elicitation effect), and then occurring again during the second half of its duration (cf. Fig. 5). This latter result resembles the inhibition-of-delay phenomenon typically observed with long conditioned stimuli in Pavlovian training (e.g., Rescorla, 1967).
With established patterns of lever pressing and schedule-induced licking, protective response–food delays permitted visualization of food delay gradients for both adjunctive licking and lever pressing, with lever pressing showing steeper gradients. Food delays had to increase to 20–25 s to start affecting the peak of the temporal distribution of licking (cf. Fig. 5), a shift that occurred with delays as short as 1 s for lever pressing. Short lick–food delays (up to 10 s) did not affect responding because licking seldom occurred during the last 10 s of the interfood intervals (see the upper left-hand panel of Fig. 5). The changes in licking and lever pressing with the introduction of delays were not attributable to changes in food frequency, since this did not change substantially with the response–food delay (cf. Fig. 4). Indeed, even at very long response–food delays food frequency was not much reduced, because pressing was almost completely abolished and licking was sufficiently distant from food to seldom postpone it. We infer that the interval between the occurrence of the response and the occurrence of food is the variable that is critical for level of responding.
When delays were discontinued at the end of the experiment, licking increased but lever pressing did not. This shows the difficulty of instantiating lever pressing by simply delivering pellets of food regularly and independently of responding. Just as licking was less sensitive to the disruptive effects of response–food delays than was lever pressing, when delays that affected licking were removed, it returned to near-baseline levels, whereas lever pressing did not. This suggests that licking has greater response strength or behavioral momentum than does lever pressing (Nevin & Grace, 2000). It could be argued that given the lack of recovery of lever pressing when protective delays were removed, lever pressing might have extinguished as delays were increased (e.g., Rescorla & Skucy, 1969). However, several details of the procedure employed here, and some of the results (such as the gradient relating the amount of lever pressing and food delay), do not support this view. Lever pressing was maintained for 20 sessions with response-independent food deliveries (see Fig. 1) prior to the delay phases, so if just delivering food independently of lever pressing were responsible for the reduction in behavior seen in those delays, such decreases should have occurred before the delays were introduced.
The results for licking are consistent with Falk’s (1964) original demonstration of the resistance of schedule-induced polydipsia to reduction by lick–food delays. In Falk’s (1964) study, only the licks occurring in the last 15 s of average 60-s interreinforcement intervals postponed food, a procedure similar to the one used here, with comparable results. The present results show negligible effects of delays shorter than 10 s in a 30-s interfood interval, a proportion of delay length to food interval similar to that of Falk (1964). Killeen (1975) found a similar resistance of pigeons’ general activity to reduction by protective response–food delays up to 12 s. Licking in the present report was not affected by protective delays of less than 20 s because licks were seldom followed by food delays. This absence of actual contact between licks and food postponement could explain what happened in Falk’s (1964) report of the failure of lick-dependent delays to reduce established schedule-induced polydipsia (see the Introduction for a more detailed presentation of this view). When postfood licking is kept distant from food reinforcement, it is reduced by that action. To further support this possibility, Pellón and Castilla (2000) found that lick-dependent delays as short as 3 or 6 s were able to reduce licking when the behavior was induced by short interfood intervals (an FT 18-s schedule), which guaranteed that postfood licking effectively postponed food delivery. Finally, Keehn and Stoyanov (1983) reported a suppression of water consumption when food was only available 50 or 60 s from the last lick (induced by a FT 60-s food schedule), a finding also consistent with what we have obtained here.
The results for lever pressing resemble delay-of-reinforcement gradients previously derived with schedule-maintained lever pressing, particularly the steep slopes derived from obtained delay values of response-dependent unsignaled delay procedures (e.g., Richards, 1981; Sizemore & Lattal, 1978). When reinforcement rates have been equated between immediate and delayed reward conditions in standard operant procedures (such as found by Chung, 1965, for key pecking in pigeons), response rates are reduced at delay values greater than 1 s, a result quantitatively comparable to the present report with lever pressing.
Changes in overall licking and lever pressing were accompanied by corresponding changes in local responding, with licking predominant during the first part of the interfood intervals, and lever pressing during the second part. This pattern of responding did not change with the introduction of delays, but merely reduced in amplitude, with lever pressing again being much more sensitive to short response–food delays than was licking. An interesting observation of the present study (not reported above) was a linear and positive relation between the occurrences of licking and lever pressing across the different experimental conditions, which reflects the co-occurrence of both responses within interfood intervals (see Ardoy & Pellón, 2004, and Reid & Dale, 1985, for theoretical analyses of such a relation).
The amount of schedule-induced drinking is related to the parameters that define the food reinforcer, such as its magnitude or quality, the rate at which it is presented, or the animal’s level of food deprivation (Pellón, 1992; Reid & Staddon, 1990). In this respect, there are no fundamental differences between schedule-induced and schedule-maintained behaviors. Schedule-induced drinking, on the contrary, does not show systematic relationships with variables related to drinking behavior itself, such as the animal’s level of thirst or the nature of the liquid available (see Pellón, 1992). Although schedule-induced polydipsia covaries more with food motivation than with water motivation, water may have ancillary reinforcing properties by making the food pellet itself more reinforcing (Keehn & Burton, 1978; Roper & Crossland, 1982).
As we detailed in the introduction, ample evidence is now showing that the rate of schedule-induced drinking is sensitive to environmental consequences programmed in relation to the rats’ licking, in a way similar to rats’ lever pressing being reinforced by food (Bond, Blackman, & Scruton, 1973; Pellón & Blackman, 1987; Reberg, 1980). Furthermore, the variables related to food that have been shown to affect adjunctive behavior also serve to modulate the effects of environmental consequences on licking. For example, Lamas and Pellón (1995b) punished schedule-induced drinking through lick-dependent food delays and varied the levels of food deprivation of their rats. When the level of food deprivation was high, the efficacy of the punishment procedure in reducing adjunctive behavior was diminished. This effect on punished schedule-induced drinking resembles similar effects of punishment procedures on food-maintained lever pressing (Azrin, Holz, & Hake, 1963).
As was outlined in the preceding paragraphs, adjunctive behavior is amenable to modification by its environmental consequences and is affected similarly to operant behavior by variables related to the food reinforcer. It remains to be determined to what extent adjunctive behavior is maintained by positive reinforcement (Pellón, 2004; Wetherington, 1982).
Clark’s (1962) suggestion that schedule-induced drinking develops because licking is adventitiously reinforced by the next food delivery was rejected on the bases that licking is normally a postpellet rather than a prepellet phenomenon, which is inconsistent with the view that superstitious behavior can only occur if behavior is followed closely by reinforcement (Skinner, 1948). Operant responses, however, can be acquired and maintained with long response–reinforcer delays (D’Amato et al., 1981; Dickinson et al., 1992; Spetch & Honig, 1988). For example, Capaldi (1978) showed acquisition by rats of running in runways with 20-s delays, and Lattal and Gleeson (1990) demonstrated the acquisition of key pecking by pigeons and lever pressing by rats with delays of 30 s. The experiments of Lattal and Gleeson are particularly relevant for the analysis of adjunctive behavior, because they were run in acquisition and without any response shaping, just as most studies of schedule-induced drinking are.
Therefore, there appears to be no conceptual obstacle for the view that superstitious behavior will be maintained by delayed as well as by immediate reinforcement, and this could also be true for adjunctive behavior (Killeen & Pellón, in press). Acquisition of adjunctive behavior is not simply a matter of accommodation to the feeding schedule, as schedule-induced drinking can be acquired even after extended pretraining with the feeding schedule (Reynierse & Spanier, 1968; S. L. Williams, Tang, & Falk, 1992). Drinking followed by delayed reinforcement is like acquisition of lever pressing with delayed reinforcement, in that both are acquired at about the same rate when reinforcements are similarly delayed (when food is not delayed, operant lever pressing is acquired faster than adjunctive licking: Ardoy & Pellón, 2004; Pellón, 2004). Additionally, that the rate of adjunctive behavior depends on the length of the interfood interval (Falk, 1966; Flores & Pellón, 1995; Flory, 1971) might be seen as analogous to the effects of response–reinforcer delays in operant conditioning: Given that licking is usually a postpellet phenomenon (e.g., Flores & Pellón, 1997), lengthening the interfood interval could be similar to delaying food for positively reinforced operant behavior.
Some other findings of the present report might also conform to results reported for operant responding. Interesting, albeit rather complex, observations are the data showing that licks decreased with 1-s delays but slightly increased with 2-s delays, in comparison to baseline levels with no delays (cf. Fig. 2). The results for 1-s delays might reflect competition between licking and lever pressing, because rats still pressed the lever as soon as it was inserted in the chamber and continued doing so throughout the interfood interval (cf. Fig. 5). Increases in licking with 2-s delays might be similar to increases obtained after very brief resetting delays (0.5 s) were imposed on operant responding (Lattal & Ziegler, 1982), with the difference that in adjunctive drinking, these delays were four orders of magnitude longer (as, in general, delays need to be much longer to have comparable effects).
An alternative (and complementary) possibility for how very long food delays might maintain adjunctive drinking is through the bridging of the lick–food interval by keeping the rats’ mouths wet due to water ingestion. Drinking in rats is associated with eating (a postprandial phenomenon: Kissileff, 1969), and is thus a predominant behavior in most interfood intervals and across animals (contrary to other observations of nonconsummatory superstitious behavior, such as in Skinner, 1948). Such a prevalence of drinking would be in line with preorganized patterns of foraging behavior (Timberlake & Lucas, 1989; see also Staddon & Simmelhag, 1971).
In summary, the present results show that delayed reinforcement might affect interim and terminal activities differentially, but through a common mechanism—namely, the temporal organization of behavior toward a common goal. This analysis is based, first, on the induction by reinforcement of preorganized patterns of behavior, and, second, on the tuning by reinforcement of activities during the interreinforcement intervals (a similar proposal has recently been made by Baum, 2012). This analysis leads to two final general reflections about reinforcement. First, the absence of explicitly arranged contingencies, such as is normal in schedule induction procedures, does not exclude the creation and maintenance of them (Lattal, 1995; Papini & Bitterman, 1990). Second, reinforcers can strengthen a pattern of behavior by increasing the probability of its constituent elements directly and for the pattern as a whole (Rachlin, 1994; Shimp, 1981), opening the possibility for researchers to conceive contingency in the more molar vein of correlation in time between aggregates of responses and reinforcers (Baum, 1973; Rachlin, 1994; B. A. Williams, 1983).
References
Ardoy, J., & Pellón, R. (2004). Effects of withholding the opportunity to press the operant lever on the maintenance of schedule-induced drinking in rats. Revista Mexicana de Análisis de la Conducta, 30, 79–91.
Azrin, N. H., Holz, W. C., & Hake, D. (1963). Fixed-ratio punishment. Journal of the Experimental Analysis of Behavior, 6, 141–148.
Baum, W. M. (1973). The correlation-based law of effect. Journal of the Experimental Analysis of Behavior, 20, 137–153.
Baum, W. M. (2012). Rethinking reinforcement: Allocation, induction, and contingency. Journal of the Experimental Analysis of Behavior, 97, 101–124.
Bond, N. W., Blackman, D. E., & Scruton, P. (1973). Suppression of operant behavior and schedule-induced licking in rats. Journal of the Experimental Analysis of Behavior, 20, 375–383.
Capaldi, E. J. (1978). Effects of schedule and delay of reinforcement on acquisition speed. Animal Learning and Behavior, 6, 330–334.
Chung, S.-H. (1965). Effects of delayed reinforcement in a concurrent situation. Journal of the Experimental Analysis of Behavior, 8, 439–444.
Clark, F. C. (1962). Some observations on the adventitious reinforcement of drinking under food reinforcement. Journal of the Experimental Analysis of Behavior, 5, 61–63.
D’Amato, M. R., Safarjan, W. R., & Salmon, D. (1981). Long-delay conditioning and instrumental leaming: Some new findings. In N. E. Spear & R. R. Miller (Eds.), Information processing in animals: Memory mechanisms (pp. 113–142). Mahwah, NJ: Erlbaum.
Davey, G. C. L., & Cleland, G. G. (1982). Topography of signal-centered behavior in the rat: Effects of deprivation state and reinforcer type. Journal of the Experimental Analysis of Behavior, 38, 291–304.
Dickinson, A., Watt, A., & Griffiths, W. J. H. (1992). Free-operant acquisition with delayed reinforcement. Quarterly Journal of Experimental Psychology, 45B, 241–258.
Falk, J. L. (1961). Production of polydipsia in normal rats by an intermittent food schedule. Science, 133, 195–196.
Falk, J. L. (1964). Studies on schedule-induced polydipsia. In M. J. Wayner (Ed.), Thirst: First International Symposium on Thirst in the Regulation of Body Water (pp. 95–116). New York, NY: Pergamon Press.
Falk, J. L. (1966). Schedule-induced polydipsia as a function of fixed-interval length. Journal of the Experimental Analysis of Behavior, 9, 37–39.
Falk, J. L. (1971). The nature and determinants of adjunctive behavior. Physiology and Behavior, 6, 577–588.
Flores, P., & Pellón, R. (1995). Rate-dependency hypothesis and the rate-decreasing effects of d-amphetamine on schedule-induced drinking. Behavioural Pharmacology, 6, 16–23.
Flores, P., & Pellón, R. (1997). Effects of d-amphetamine on temporal distributions of schedule-induced polydipsia. Pharmacology, Biochemistry and Behavior, 57, 81–87.
Flory, R. K. (1971). The control of schedule-induced polydipsia: Frequency and magnitude of reinforcement. Learning and Motivation, 2, 215–227.
Flory, R. K., & Lickfett, G. G. (1974). Effects of lick-contingent timeout on schedule-induced polydipsia. Journal of the Experimental Analysis of Behavior, 21, 45–55.
Hawkins, T. D., Schrot, J. F., Githens, S. H., & Everett, P. B. (1972). Schedule-induced polydipsia: An analysis of water and alcohol ingestion. In R. M. Gilbert & J. D. Keehn (Eds.), Schedule effects: Drugs, drinking and aggression (pp. 95–128). Toronto, ON: University of Toronto Press.
Keehn, J. D., & Burton, M. (1978). Schedule-induced drinking: Entrainment by fixed-and random-interval schedule-controlled feeding. T.-I.-T. Journal of Life Sciences, 8, 93.
Keehn, J. D., & Stoyanov, E. (1983). Disruption of adjunctive drinking by lick-dependent delays in feeding. Psychological Record, 33, 391–400.
Killeen, P. (1975). On the temporal control of behavior. Psychological Review, 82, 89–115. doi:10.1037/h0076820
Killeen, P. R., & Pellón, R. (in press). Adjunctive behaviors are operants. Learning and Behavior.
Kissileff, H. R. (1969). Food-associated drinking in the rat. Journal of Comparative and Physiological Psychology, 67, 284–300.
Lamas, E., & Pellón, R. (1995a). Food-delay duration and the development of schedule-induced polydipsia in rats. Physiology and Behavior, 57, 1221–1224.
Lamas, E., & Pellón, R. (1995b). Food-deprivation effects on punished schedule-induced drinking in rats. Journal of the Experimental Analysis of Behavior, 64, 47–60.
Lamas, R., & Pellón, R. (1997). Food deprivation and food-delay effects on the development of adjunctive drinking. Physiology and Behavior, 61, 153–158.
Lashley, R. L., & Rosellini, R. A. (1980). Modulation of schedule-induced polydipsia by Pavlovian conditioned states. Physiology and Behavior, 24, 411–414.
Lattal, K. A. (1995). Contingency and behavior analysis. Behavior Analyst, 18, 209–224.
Lattal, K. A. (2010). Delayed reinforcement of operant behavior. Journal of the Experimental Analysis of Behavior, 93, 129–139.
Lattal, K. A., & Gleeson, S. (1990). Response acquisition with delayed reinforcement. Journal of Experimental Psychology: Animal Behavior Processes, 16, 27–39.
Lattal, K. A., & Ziegler, D. R. (1982). Briefly delayed reinforcement: An interresponse time analysis. Journal of the Experimental Analysis of Behavior, 37, 407–416.
Locurto, C., Terrace, H. S., & Gibbon, J. (1976). Autoshaping, random control and omission training in the rat. Journal of the Experimental Analysis of Behavior, 26, 451–462.
Moran, G., & Rudolph, R. L. (1980). Some effects of lick-contingent delays on the development of schedule-induced polydipsia. Learning and Motivation, 11, 366–385.
Nevin, J. A., & Grace, R. C. (2000). Behavioral momentum and the law of effect. Behavioral and Brain Sciences, 23, 73–130.
Papini, M. R., & Bitterman, M. E. (1990). The role of contingency in classical conditioning. Psychological Review, 97, 396–403. doi:10.1037/0033-295X.97.3.396
Pellón, R. (1992). Polidipsia inducida por programa: II. Variables motivacionales [Schedule-induced polydipsia: II. Motivational variables]. Revista de Psicología: General y Aplicada, 45, 251–265.
Pellón, R. (2004). La ley del efecto y la conducta innata [The law of effect and innate behavior]. In R. Pellón & A. Huidobro (Eds.), Inteligencia y aprendizaje (pp. 89–114). Barcelona, Spain: Ariel.
Pellón, R., & Blackman, D. E. (1987). Punishment of schedule-induced drinking in rats by signaled and unsignaled delays in food presentation. Journal of the Experimental Analysis of Behavior, 48, 417–434.
Pellón, R., & Blackman, D. E. (1991). The effects of signalled and unsignalled lick-dependent delays on the development of schedule-induced drinking in rats. Quarterly Journal of Experimental Psychology, 43B, 39–57.
Pellón, R., & Castilla, J. L. (2000). Punishment of schedule-induced drinking by lick-dependent delays in food presented at different frequencies. Psychological Record, 50, 141–153.
Rachlin, H. (1994). Behavior and mind. New York, NY: Oxford University Press.
Reberg, D. (1980). Reinforcing the occurrence or non-occurrence of interim drinking. Animal Learning and Behavior, 8, 120–128.
Reid, A. K., & Dale, R. H. I. (1985). Dynamic effects of food magnitude on interim-terminal interaction. Journal of the Experimental Analysis of Behavior, 39, 135–148.
Reid, A., & Staddon, J. E. R. (1990). Mechanisms of schedule entrainment. In S. J. Cooper & C. T. Dourish (Eds.), Neurobiology of stereotyped behavior (pp. 200–231). Oxford, U.K.: Oxford University Press, Clarendon Press.
Rescorla, R. A. (1967). Inhibition of delay in Pavlovian fear conditioning. Journal of Comparative and Physiological Psychology, 64, 114–120.
Rescorla, R. A., & Skucy, J. C. (1969). Effect of response-independent reinforcer during extinction. Journal of Comparative and Physiological Psychology, 67, 381–389.
Reynierse, J. H., & Spanier, D. (1968). Excessive drinking in rats’ adaptation to the schedule of feeding. Psychonomic Science, 10, 95–96.
Richards, R. W. (1981). A comparison of signaled and unsignaled delay of reinforcement. Journal of the Experimental Analysis of Behavior, 35, 145–152.
Roper, T. J., & Crossland, G. (1982). Schedule-induced wood-chewing in rats and its dependence on body weight. Animal Learning and Behavior, 10, 65–71.
Segal, E. F., & Oden, D. L. (1969). Schedule-induced polydipsia: Effects of providing an alternate reinforced response and of introducing a lick-contingent delay in food delivery. Psychonomic Science, 15, 153–154.
Shimp, C. P. (1981). The local organization of behavior: Discrimination of and memory for simple behavioral patterns. Journal of the Experimental Analysis of Behavior, 36, 303–315.
Sizemore, O. J., & Lattal, K. A. (1978). Unsignaled delay of reinforcement in variable-interval schedules. Journal of the Experimental Analysis of Behavior, 30, 169–175.
Skinner, B. F. (1948). “Superstition” in the pigeon. Journal of Experimental Psychology, 38, 168–172.
Spetch, M. L., & Honig, W. K. (1988). Characteristics of pigeons’ spatial working memory in an open-field task. Learning and Behavior, 16, 123–131.
Staddon, J. E. R. (1977). Schedule-induced behavior. In W. K. Honig & J. E. R. Staddon (Eds.), Handbook of operant behavior (pp. 125–152). Englewood Cliffs, NJ: Prentice-Hall.
Staddon, J. E. R., & Simmelhag, V. L. (1971). The “superstition” experiment: A reexamination of its implications for the principles of adaptive behavior. Psychological Review, 78, 3–43.
Timberlake, W., & Lucas, G. A. (1989). Behavior systems and learning: From misbehavior to general principles. In S. B. Klein & R. R. Mowrer (Eds.), Contemporary learning theories: Instrumental conditioning theories and the impact of biological constraints on learning (pp. 237–275). Hillsdale, NJ: Erlbaum.
Wetherington, C. L. (1982). Is adjunctive behavior a third class of behavior? Neuroscience and Biobehavioral Reviews, 6, 329–350.
Williams, B. A. (1983). Revising the principle of reinforcement. Behaviorism, 11, 63–85.
Williams, S. L., Tang, M., & Falk, J. L. (1992). Prior exposure to a running wheel and scheduled food attenuates polydipsia acquisition. Physiology and Behavior, 52, 481–483.
Author note
Research and the preparation of the manuscript were supported by Spanish Government Research Grant Nos. PSI2008-03660 (Ministerio de Ciencia e Innovación: Secretaría de Estado de Investigación) and PSI2011-29399 (Ministerio de Economía y Competitividad: Secretaría de Estado de Investigación, Desarrollo e Innovación) to R.P. Ángeles Pérez Padilla was the research technician during the course of the project. Thanks to Peter R. Killeen for discussions on many of the ideas presented in this article and for corrections on a previous version.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pellón, R., Pérez-Padilla, Á. Response–food delay gradients for lever pressing and schedule-induced licking in rats. Learn Behav 41, 218–227 (2013). https://doi.org/10.3758/s13420-012-0099-x
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13420-012-0099-x