Abstract
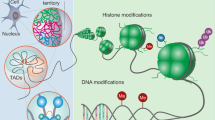
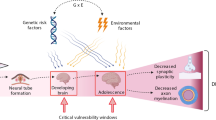
While a distinct minicolumnar phenotype seems to be an underlying factor in a significant portion of cases of autism, great attention is being paid not only to genetics but to epigenetic factors which may lead to development of the conditions. Here we discuss the indivisible role the molecular environment plays in cellular function, particularly the pivotal position which the transcription factor and adhesion molecule, β-catenin, occupies in cellular growth. In addition, the learning environment is not only integral to postnatal plasticity, but the prenatal environment plays a vital role during corticogenesis, neuritogenesis, and synaptogenesis as well. To illustrate these points in the case of autism, we review important findings in genetics studies (e.g., PTEN, TSC1/2, FMRP, MeCP2, Neurexin-Neuroligin) and known epigenetic factors (e.g., valproic acid, estrogen, immune system, ultrasound) which may predispose towards the minicolumnar and connectivity patterns seen in the conditions, showing how one-gene mutational syndromes and exposure to certain CNS teratogens may ultimately lead to comparable phenotypes. This in turn may shed greater light on how environment and complex genetics combinatorially give rise to a heterogenetic group of conditions such as autism.
Similar content being viewed by others
References
Liu J., Nyholt D.R., Magnussen P., Parano E., Pavone P., Geschwind D., et al., A genomewide screen for autism susceptibility loci, Am. J. Hum. Genet., 2001, 69, 327–340
Yonan A.L., Alarcón M., Cheng R, Magnusson P.K., Spence S.J., Palmer A.A., et al., A genomewide screen of 345 families for autism-susceptibility loci, Am. J. Hum. Genet., 2003, 73, 886–897
Williams E.L., Casanova M.F., Autism or autisms? Finding the lowest common denominator, Bol. Asoc. Méd. P.R., 2010 Oct, 102(4), 17–24
Minshew N.J., Williams D.L., The new neurobiology of autism: Cortex, connectivity, and neuronal organization, Arch. Neurol., 2007, 64, 945–950
Casanova M.F., Buxhoeveden D.P., Switala A.E., Roy E. Minicolumnar pathology in autism, Neurology, 2002, 58, 428–432
Chenn A., Walsh C.A., Regulation of cerebral cortical size by control of cell cycle exit in neural precursors, Science, 2002, 297, 365–369
Bauman M.L., Kemper T.L., Neuroanatomic observations of the brain in autism: A review and future directions, Int. J. Dev. Neurosci., 2005, 23, 183–187
Herbert M.R., Ziegler D.A., Makris N., Filipek P.A., Kemper T.L., Normandin J.J., et al., Localization of white matter volume increase in autism and developmental language disorder, Ann. Neurol., 2004, 55, 530–540
Rinaldi T., Kulangara K., Antoniello K., Markram H., Elevated NMDA receptor levels and enhanced postsynaptic long-term potentiation induced by prenatal exposure to valproic acid, Proc. Natl. Acad. Sci. U.S.A., 2007, 104, 13501–13506
Rinaldi T., Perrodin C., Markram H., Hyper-connectivity and hyperplasticity in the medial prefrontal cortex in the valproic acid animal model of autism, Front. Neural Circuits, 2008, 2, 1–7
Casanova M.F., El-Baz A., Mott M., Mannheim G., Hassan H., Fahmi R., et al., Reduced gyral window and corpus callosum size in autism: Possible macroscopic correlates of a minicolumnopathy, J. Autism Dev. Disord., 2009, 39, 751–764
Beaudet A.L., Autism: highly heritable but not inherited, Nat. Med., 2007, 13, 534–536
Muhle R., Trentacoste S.V., Rapin I., The genetics of autism, Pediatrics, 2004, 113, e472–e486
Herbert M.R., Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders, Curr. Opin. Neurol., 2010, 23, 103–110
Courchesne E., Carper R., Akshoomoff N., Evidence of brain overgrowth in the first year of life in autism, J. Am. Med. Assoc., 2003, 290, 337–344
Rogers S.J., Developmental regression in autism spectrum disorders, Ment. Retard. Dev. Disabil. Res. Rev., 2004, 10, 139–143
Kumar V., Zhang M.X., Swank M.W., Kunz J., Wu G.Y., Regulation of dendritic morphogenesis by Ras-PI3K-Akt-mTOR and Ras-MAPK signaling pathways, J. Neurosci., 2005, 25, 11288–11299
McDaniel M.A., Big-brained people are smarter: A meta-analysis of the relationship between in vivo brain volume and intelligence, Intelligence, 2005, 33, 337–346
Burrell B., Postcards from the brain museum, Broadway Books, New York, 2004
Happé F., Frith U., The weak central coherence account: Detail-focused cognitive style in autism spectrum disorders, J. Autism Dev. Disord., 2006, 36, 5–25
Treffert D.A., Extraordinary people: Understanding savant syndrome, iUniverse, Lincoln, 2006
Redcay E., Courchesne E., When is the brain enlarged in autism? A meta-analysis of all brain size reports, Biol. Psychiatry, 2005, 58, 1–9
Pilarsky R., Cowden syndrome: A critical review of the clinical literature, J. Genet. Couns., 2009, 18, 13–27
McBride K.L., Varga E.A., Pastore M.T., Prior T.W., Manickam K, Atkin J.F., et al., Confirmation of study of PTEN mutations among individuals with autism or developmental delays/mental retardation and macrocephaly, Biol. Autism Res., 2010, 3, 137–141
Tamguney T., Stokoe D., New insights into PTEN, J. Cell. Sci., 2007, 120, 4071–4079
Nan X., Ng H.H., Johnson C.A., Laherty C.D., Turner B.M., Eisenman R.N., et al., Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex, Nature, 1998, 393, 386–389
Muotri A.R., Marchetto M.C., Coufal N.G., Oefner R., Yeo G., Nakashima K, et al., L1 retrotransposition in neurons is modulated by MeCP2, Nature, 2010, 468, 443–446
Skene P.J., Illingworth R.S., Webb S., Kerr A.R., James K.D., Turner D.J., et al., Neuronal MeCP2 is expressed at near histone-octamer levels and globally alters the chromatin state, Mol. Cell, 2010, 37, 457–468
Nelson W.J., Nusse R., Convergence of Wnt, β-catenin, and cadherin pathways, Science, 2004, 303, 1483–1487
Persad S., Troussard A.A., McPhee T.R., Mulholland D.J., Dedhar S., Tumor suppressor PTEN inhibits nuclear accumulation of beta-catenin and T cell/lymphoid enhancer factor 1-mediated transcriptional activation, J. Cell Biol., 2001, 153, 1161–1174
Carney R.M., Wolpert C.M., Ravan S.A., Shahbazian M., Ashley-Koch A., Cuccaro M.L., et al., Identification of MeCP2 mutations in a series of females with autistic disorder, Pediatr. Neurol., 2003, 28, 205–211
Samaco R.C., Nagarajan R.P., Braunschweig D., LaSalle J.M., Multiple pathways regulate MeCP2 expression in normal brain development and exhibit defects in autism-spectrum disorders, Hum. Mol. Genet., 2004, 13, 629–639
Steelman L.S., Abrams S.L., Whelan J., Bertrand F.E., Ludwig D.E., Bäsecke J., et al., Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia, Leukemia, 2008, 22, 686–707
Kim D.H., Sarbassov D.D., Ali S.M., King J.E., Latek R.R., Erdjument-Bromage H., et al., mTOR interacts with raptor to form a nutrientsensitive complex that signals to the cell growth machinery, Cell, 2002, 110, 163–175
Wiznitzer M., Autism and tuberous sclerosis, J. Child Neurol., 2004, 19, 675–679
Ehninger D., De Vries P.J., Silva A.J., From mTOR to cognition: Molecular and cellular mechanisms of cognitive impairments in tuberous sclerosis, J. Intellect. Disabil. Res., 2009, 53, 838–851
Griffiths P.D., Gardner S.A., Smith M., Rittey C., Powell T., Hemimegalencephaly and focal megalencephaly in tuberous sclerosis complex, Am. J. Neuroradiol., 1998, 19, 1935–1938
Christophe C., Sékhara T., Rypens F., Ziereisen F., Christiaens F., Dan B., MRI spectrum of cortical malformations in tuberous sclerosis complex, Brain Dev., 2000, 22, 487–493
Way S.W., McKenna J. 3rd, Mietzsch U., Reith R.M., Wu H.C., Gambello M.J., Loss of Tsc2 in radial glia models the brain pathology of tuberous sclerosis complex in the mouse, Hum. Mol. Genet., 2009, 18, 1252–1265
Bailey A., Luthert P., Dean A., Harding B., Janota I., Montgomery M., et al., A clinicopathological study of autism, Brain, 1998, 121, 889–905
Wegiel J., Kuchna I., Nowicki K., Imaki H., Wegiel J., Marchi E., et al., The neuropathology of autism: Defects of neurogenesis and neuronal migration, and dysplastic changes, Acta Neuropathol., 2010, 119, 755–770
Mak B.C., Takemaru K., Kenerson H.L., Moon R.T., Yeung R.S., The tuberin-hamartin complex negatively regulates beta-catenin signaling activity, J. Biol. Chem., 2003, 278, 5947–5951
Daugherty R.L., Gottardi C.J., Phospho-regulation of β-catenin adhesion and signaling functions, Physiology, 2007, 22, 303–309
Brown V., Jin P., Ceman S., Darnell J.C., O’Donnell W.T., Tenenbaum S.A., et al., Microarray identification of FMRP-associated brain mRNAs and altered mRNA translational profiles in fragile X syndrome, Cell, 2001, 107, 477–487
Luo Y., Shan G., Guo W., Smrt R.D., Johnson E.B., Li X., et al., Fragile X mental retardation protein regulates proliferation and differentiation of adult neural stem/progenitor cells, PLoS Genet., 2010, 6, e1000898
Hagerman R.J., Fragile X syndrome, In: Bauman M.L., Kemper T.L. (Eds.), The neurobiology of autism, 2nd ed., The Johns Hopkins University Press, London, 2005, 251–264
Fatemi S.H., Folsom T.D., The role of fragile X mental retardation protein in major mental disorders, Neuropharmacology, 2011, 60, 1221–1226
Zalfa F., Marcello G., Primerano B., Moro A., Di Penta A., Reis S., et al., The fragile X syndrome protein FMRP associates with BC1 RNA and regulates the translation of specific mRNAs at synapses, Cell, 2003, 112, 317–327
Castrén M., Tervonen T., Kärkkäinen V., Heinonen S., Castrén E., Larsson K., et al., Altered differentiation of neural stem cells in fragile X syndrome, Proc. Natl. Acad. Sci. U.S.A., 2005, 102, 17834–17839
Tervonen T.A., Louhivuori V., Sun X., Hokkanen M.E., Kratochwil C.F., Zebryk P., et al., Aberrant differentiation of glutamatergic cells in neocortex of mouse model for fragile X syndrome, Neurobiol. Dis., 2009, 33, 250–259
De Vries B.B.A., Mohkamsing S., Van den Ouweland A.M.W., Mol E., Gelsema K., Van Rijn M., et al., Screening for the fragile X syndrome among the mentally retarded: a clinical study, J. Med. Genet., 1999, 36, 467–470
Chausovsky A., Bershadsky A.D., Borisy G.G., Cadherin-mediated regulation of microtubule dynamics, Nat. Cell Biol., 2000, 2, 797–804
Reynolds A.B., Daniel J., McCrea P.D., Wheelock M.J., Wu J., Zhang Z., Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes, Mol. Cell Biol., 1994, 14, 8333–8342
Bienz M., β-catenin: A pivot between cell adhesion and Wnt signalling, Curr. Biol., 2004, 15, R65
Ziegler S., Röhrs S., Tickenbrock L., Möröy T., Klein-Hitpass L., Vetter I.R., et al., Novel target genes of the Wnt pathway and statistical insights into Wnt target promoter regulation, FEBS J., 2005, 272, 1600–1615
Gearhart J., Pashos E.E., Prasad M.K., Pluripotency redux—advances in stem-cell research, N. Engl. J. Med., 2007, 357, 1469–1472
Cotterman R., Jin V.X., Krig S.R., Lemen J.M., Wey A., Farnham P.J., et al., N-Myc regulates a widespread euchromatic program in the human genome partially independent of its role as a classic transcription factor, Cancer Res., 2008, 68, 9654–9662
Nusse R., A list of target genes of Wnt/beta-catenin signaling [online resource], Howard Hughes Medical Center, Stanford, 2009 [accessed 2011 Jan 28], http://www.stanford.edu/~rnusse/pathways/targets.html
Ding Q., Xia W., Liu J.C., Yang J.Y., Lee D.F., Xia J., et al., Erk associates with and primes GSK-3β for its inactivation resulting in upregulation of β-catenin, Mol. Cell, 2005, 19, 159–170
Gherzi R., Trabucchi M., Ponassi M., Ruggiero T., Corte G., Moroni C., et al., The RNA-binding protein KSRP promotes decay of betacatenin mRNA and is inacitvated by PI3K-AKT signaling, PLoS Biol., 2006, 5, e5
Bamji S.X., Shimazu K., Kimes N., Huelsken J., Birchmeier W., Lu B., et al., Role of beta-catenin in synaptic vesicle localization and presynaptic assembly, Neuron, 2003, 40, 719–731
Kwon C.H., Luikart B.W., Powell C.M., Zhou J., Matheny S.A., Zhang W., et al., Pten regulates neuronal arborization and social interaction in mice, Neuron, 2006, 50, 377–388
Wang Y., Greenwood J.S., Calcagnotto M.E., Kirsch H.E., Barbaro N.M., Baraban S.C., Neocortical hyperexcitability in a human case of tuberous sclerosis complex and mice lacking neuronal expression of TSC1, Ann. Neurol., 2007, 61, 139–152
Nau H., Rating D., Koch S., Häuser I., Helge H., Valproic acid and its metabolites: Placental transfer, neonatal pharmacokinetics, transfer via mother’s milk and clinical status in neonates of epileptic mothers, J. Pharmacol. Exp. Ther., 1981, 219, 768–777
DiLiberty J.H., Farndon P.A., Dennis N.R., Curry C.J., The fetal valproate syndrome, Am. J. Med. Genet., 1984, 19, 473–481
Christianson A.L., Chesler N., Kromberg J.G., Fetal valproate syndrome: Clinical and neuro-developmental features in two sibling pairs, Dev. Med. Child Neurol., 1994, 36, 361–369
Moore S.J., Turnpenny P., Quinn A., Glover S., Lloyd D.J., Montgomery T., et al., A clinical study of 57 children with fetal anticonvulsant syndromes, J. Med. Genet., 2000, 37, 489–497
Rasalam A.D., Hailey H., Williams J.H., Moore S.J., Turnpenny P.D., Lloyd D.J., et al., Characteristics of fetal anticonvulsant syndrome associated autistic disorder, Dev. Med. Child Neurol., 2005, 47, 551–555
Markram H., Rinaldi T., Markram K.. The intense world syndrome—an alternative hypothesis for autism, Front. Neurosci., 2007, 1, 77–96
Shimshoni J.A., Dalton E.C., Jenkins A., Eyal S., Ewan K., Williams R.S., et al., The effects of central nervous system-active valproic acid constitutional isomers, cyclopropyl analogs, and amide derivatives on neuronal growth cone behavior, Mol. Pharmacol., 2007, 71, 884–892
Billin A.N., Thirlwell H., Ayer D.E., β-catenin-histone deacetylase interactions regulate the transition of LEF1 from a transcriptional repressor to an activator, Mol. Cell Biol., 2000, 20, 6882–6890
Wiltse J., Mode of action: inhibition of histone deacetylase, altering WNT-dependent gene expression, and regulation of beta-catenin—developmental effects of valproic acid, Crit. Rev. Toxicol., 2005, 35, 727–738
Wang Z., Xu L., Zhu X., Cui W., Sun Y., Nishijo H., et al., Demethylation of specitic Wnt/β-catenin pathway genes and its upregulation in rat brain induced by prenatal valproate exposure, Anat. Rec., 2010, 293, 1947–1953
Raballo R., Rhee J., Lyn-Cook R., Leckman J.F., Schwartz M.L., Vaccarino F.M., Basic fibroblast growth factor (Fgf2) is necessary for cell proliferation and neurogenesis in the developing cerebral cortex., J. Neurosci., 2000, 20, 5012–5023
Ryves J.W., Dalton E.C., Harwood A.J., Williams R.S., GSK-3 activity in neocortical cells is inhibited by lithium but not carbamazepine or valproic acid, Bipolar Disord., 2005, 7, 260–265
Jope R.S., Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes, Trends Pharmacol. Sci., 2003, 24, 441–443
Yuskaitis C.J., Mines M.A., King M.K., Sweatt J.D., Miller C.A., Jope R.S., Lithium ameliorates altered glycogen synthase kinase-3 and behavior in a mouse model of fragile X syndrome, Biochem. Pharmacol., 2010, 79, 632–646
Hashimoto R., Senatorov V., Kanai H., Leeds P., Chuang D.M., Lithium stimulates progenitor proliferation in cultured brain neurons, Neuroscience, 2003, 117, 55–61
Laeng P., Pitts R.L., Pemire A.L., Drabik C.E., Weiner A., Tang H., et al., The mood stabilizer valproic acid stimulates GABA neurogenesis from rat forebrain stem cells, J. Neurochem., 2004, 91, 238–251
Vecsler M., Simon A.J., Amariglio N., Rechavi G., Gak E., MeCP2 deficiency downregulates specific nuclear proteins that could be partially recovered by valproic acid in vitro, Epigenetics, 2010, 5, 61–67
Tropea D., Giacometti E, Wilson N.R., Beard C., McCurry C., Fu D.D., et al., Partial reversal of Rett syndrome-like symptoms in MeCP2 mutant mice, Proc. Natl. Acad. Sci. U.S.A., 2009, 106, 2029–2034
McCaffrey P., Deustch C.K., Macrocephaly and the control of brain growth in autistic disorders, Prog. Neurobiol., 2005, 77, 38–56
Croen L.A., Goines P., Braunschweig D., Yolkne R., Yoshida C.K., Grether J.K., et al., Brain-derived neurotrophic factor and autism: Maternal and infant peripheral blood levels in the Early Markers for Autism (EMA) study, Autism Res., 2008, 1, 130–137
Vaccarino F.M., Grigorenko E.L., Smith K.M., Stevens H.E., Regulation of cerebral cortical size and neuron number by fibroblast growth factors: Implications for autism, J. Autism Dev. Disord., 2009, 39, 511–520
Baron-Cohen S., The extreme male brain theory of autism, Trends Cogn. Sci., 2002, 6, 248–254
Knickmeyer R., Baron-Cohen S., Raggatt P., Taylor K., Hackett G., Fetal testosterone and empathy, Horm. Behav., 2006, 49, 282–292
Knickmeyer R., Baron-Cohen S., Fane B.A., Wheelwright S., Mathews G.A., Conway G.S., et al., Androgens and autistic traits: a study of individuals with congenital adrenal hyperplasia, Horm. Behav., 2006, 50, 148–153
Hague W.M., Adams J., Rodda C., Brook C.G., De Bruyn R., Grant D.B., et al., The prevalence of polycystic ovaries in patients with congenital adrenal hyperplasia and their close relatives, Clin. Endocrinol., 1990, 33, 501–510
Ingudomnukul E., Baron-Cohen S., Wheelwright S., Knickmeyer R., Elevated rates of testosterone-related disorders in women with autism spectrum conditions, Horm. Behav., 2007, 51, 597–604
Shayya R., Chang R.J., Reproductive endocrinology of adolescent polycystic ovary syndrome, BJOG, 2010, 117, 150–155
Yang F., Li X., Sharma M., Sasaki C.Y., Longo D.L., Lim B., et al., Linking beta-catenin to androgen-signaling pathway, J. Biol. Chem., 2002, 277, 11336–11344
Pawlowski J.E., Ertel J.R., Allen M.P., Xu M., Butler C., Wilson E.M., et al., Liganded androgen receptor interaction with beta-catenin: Nuclear co-localization and modulation of transcriptional activity in neuronal cells, J. Biol. Chem., 2002, 277, 20702–20710
Cullen D.A., Killick R., Leigh P.N., Gallo J.M., The effect of polyglutamine expansion in the human androgen receptor on its ability to suppress β-catenin-Tcf/Lef dependent transcription, Neurosci. Lett., 2004, 354, 54–58
MacLusky N.J., Clark A.S., Naftolin F., Goldman-Rakic P.S., Estrogen formation in the mammalian brain: Possible role of aromatase in sexual differentiation of the hippocampus and neocortex, Steroids, 1987, 50, 459–474
Lemmen J.G., Broekhof J.L.M., Kuiper G.G.J.M., Gustafsson J.Å., van der Saag P.T., van der Burg B., Expression of estrogen receptor alpha and beta during mouse embryogenesis, Mech. Dev., 1999, 81, 163–167
Forlano P.M., Deitcher D.L., Myers D.A., Bass A.H., Anatomical distribution and cellular basis for high levels of aromatase activity in the brain of teleost fish: Aromatase enzyme and mRNA expression identify glia as source, J. Neurosci., 2001, 21, 8943–8955
Wang L., Andersson S., Warner M., Gustafsson J.A., Estrogen receptor (ER)beta knockout mice reveal a role for ERbeta in migration of cortical neurons in the developing brain, Proc. Natl. Acad. Sci. U.S.A., 2003, 100, 703–708
Pardridge W.M., Mietus L.J., Transport of steroid hormones through the rat blood-brain barrier, J. Clin. Invest., 1979, 64, 145–154
Cardona-Gomez P., Perez M., Avila J., Garcia-Segura L.M., Wandosell F., Estradiol inhibits GSK3 and regulates interaction of estrogen receptors, GSK3, and beta-catenin in the hippocampus, Mol. Cell. Neurosci., 2004, 25, 363–373
Perez-Martin M., Azcoitia I., Trejo J.L., Sierra A., Garcia-Segura L.M., An antagonist of estrogen receptors blocks the induction of adult neurogenesis by insulin-like growth factor-I in the dentate gyrus of adult female rat, Eur. J. Neurosci., 2003, 18, 923–930
Homburg R., Pariente C., Lunenfeld B., Jacobs H.S., The role of insulin-like growth factor-1 (IGF-1) and IGF binding protein-1 (IGFBP-1) in the pathogenesis of polycystic ovary syndrome, Hum. Reprod., 1992, 7, 1379–1383
Kouzmenko A.P., Takeyama K., Ito S., Furatani T., Sawatsubashi S., Maki A., et al., Wnt/β-catenin and estrogen signaling converge in vivo, J. Biol. Chem., 2004, 279, 40255–40258
Varea O., Garrido J.J., Dopazo A., Mendex P., Garcia-Segura L.M., Wandosell F., Estradiol activates beta-catenin dependent transcription in neurons, PLoS ONE, 2009, 4, e5153
Simoncini T., Hafezi-Mghadam A., Brazil D.P., Ley K., Chin W.W., Liao J.K., Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase, Nature, 2000, 407, 538–541
Kuiper G.G., Carlsson B., Grandien K., Enmark E., Häggblad J., Nilsson S., et al., Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta, Endocrinology, 1997, 138, 863–870
Martin J.T., Sexual dimorphism in immune function: The role of prenatal exposure to androgens and estrogens, Eur. J. Pharmacol., 2000, 405, 251–261
Warren R.P., Odell J.D., Warren W.L., Burger R.A., Maciulis A, Daniels W.W., et al., Brief report: Immunoglobulin A deficiency in a subset of autistic subjects, J. Autism Dev. Disord., 1997, 27, 187–192
Gupta S., Aggarwal S., Rashanravan B., Lee T., Th1- and Th2-like cytokines in CD4+ and CD8+ T cells in autism, J. Neuroimmunol., 1998, 85, 106–109
Ashwood P., Van de Water J., Is autism an autoimmune disease? Autoimmun. Rev., 2004, 3, 557–562
Li X., Chauhan A., Sheikh A.M., Patil S., Chauhan V., Li X.M., et al., Elevated immune response in the brain of autistic patients, J. Neuroimmunol., 2009, 207, 111–116
Singh V.K., Phenotypic expression of autoimmune autistic disorder (AAD): A major subset of autism, Ann. Clin. Psychiatry, 2009, 21, 148–161
Grether J.K., Croen L.A., Anderson M.C., Nelson K.B., Yolken R.H., Neonatally measured immunoglobulins and risk of autism, Autism Res., 2010, 3, 323–332
Angelidou A., Alysandratos K.D., Asadi S., Zhang B., Francis K., Vasiadi M., et al., Brief report: “Allergic symptoms” in children with autism spectrum disorders. More than meets the eye? J. Autism Dev. Disord., (in press), DOI: 10.1007/s10803-010-1171-z
Chess S., Fernandez P., Korn S., Behavioral consequences of congenital rubella, J. Pediatr., 1978, 93, 699–703
Taga T., Fukuda S., Role of IL-6 in the neural stem cell differentiation, Clin. Rev. Allergy Immunol., 2005, 28, 249–256
Carpentier P.A., Palmer T.D., Immune influence on adult neural stem cell regulation and function, Neuron, 2009, 64, 79–92
Wolf S.A., Steiner B., Wengner A., Lipp M., Kammertoens T., Kempermann G., Adaptive peripheral immune response increases proliferation of neural precursor cells in the adult hippocampus, FASEB J., 2009, 23, 3121–3128
Sarkar P., Bergman K., O’Connor T.G., Glover V., Maternal antenatal anxiety and amniotic fluid cortisol and testosterone: Possible implications for foetal programming, J. Neuroendocrinol., 2008, 20, 489–496
Pascual R., Ebner D., Araneda R., Urqueta M.J., Bustamante C., Maternal stress induces long-lasting Purkinje cell developmental impairments in mouse offspring, Eur. J. Pediatr., 2010, 169, 1517–1522
You J.J., Alter D.A., Stukel T.A., McDonald S.D., Laupacis A., Liu Y., et al., Proliferation of prenatal ultrasound, Can. Med. Assoc. J., 2010, 182, 143–151
Miller M.W., Brayman A.A., Abramowicz J.S., Obstetric ultrasonography: a biophysical consideration of patient safety-the “rules” have changed, Am. J. Obstet. Gynecol., 1998, 179, 241–254
Sheiner E., Shoham-Vardi I., Abramowicz J.S., What do clinical users know regarding safety of ultrasound during pregnancy? J. Ultrasound Med., 2007, 26, 319–325
Williams E.L., Casanova M.F., Potential teratogenic effects of ultrasound on corticogenesis: Implications for autism, Med. Hypotheses, 2010, 75, 53–58
Dyson M., Franks C., Suckling J., Stimulation of healing of varicose ulcers by ultrasound, Ultrasonics, 1976, 14, 232–236
Duarte L.R., The stimulation of bone growth by ultrasound, Arch. Orthop. Trauma Surg., 1983, 101, 153–159
Ang E.S. Jr, Gluncic V., Duque A., Schafer M.E., Rakic P., Prenatal exposure to ultrasound waves impacts neuronal migration in mice, Proc. Natl. Acad. Sci. U.S.A., 2006, 103, 12903–12910
Sikov M.R., Effects of ultrasound on development. Part 2: Studies in mammalian species and overview, J. Ultrasound Med., 1986, 5, 651–661
Olkku A., Leskinen J.J., Lammi M.J., Hynynen K., Mahonen A., Ultrasound-induced activation of Wnt signaling in human MG-63 osteoblastic cells, Bone, 2010, 47, 320–330
Takeuchi R., Ryo A., Komitsu N., Mikuni-Takagaki Y., Fukui A., Takagi Y., et al., Low-intensity pulsed ultrasound activates the phosophatidylinositol 3 kinase/Akt pathway and stimulates the growth of chondrocytes in three-dimensional cultures: A basic science study, Arthritis Res. Ther., 2008, 10, R77
Mitragotri S., Blankschtein D., Langer R., Ultrasound-mediated transdermal protein delivery, Science, 1995, 269, 850–853
Van Wamel A., Bouakaz A., Versluis M., De Jong N., Micromanipulation of endothelial cells: Ultrasound-microbubble-cell interaction, Ultrasound Med. Biol., 2004, 30, 1255–1258
VanBavel E., Effects of shear stress on endothelial cells: Possible relevance for ultrasound applications, Prog. Biophys. Mol. Biol., 2007, 93, 374–383
Colombo A., Hall P., Nakamura S., Almagor Y., Maiello L., Martini G., et al., Intracoronary stenting without anticoagulation accomplished with intravascular ultrasound guidance, Circulation, 1995, 91, 1676–1688
Rioufol G., Finet G., Ginon I., André-Fouët X., Rossi R., Vialle E., et al., Multiple atherosclerotic plaque rupture in acute coronary syndrome: A three-vessel intravascular ultrasound study, Circulation, 2002, 106, 804–808
Yamamoto K., Takahashi T., Asahara T., Ohura N., Sokabe T., Kamiya A., et al., Proliferation, differentiation, and tube formation by endothelial progenitor cells in response to shear stress, J. Appl. Physiol., 2003, 95, 2081–2088
Reher P., Doan N., Bradnock B., Meghji S., Harris M., Effect of ultrasound on the production of IL-8, basic FGF and VEGF, Cytokine, 1999, 11, 416–423
Reher P., Harris M., Whiteman M., Hai H.K., Meghji S., Ultrasound stimulates nitric oxide and prostaglandin E2 production by human osteoblasts, Bone, 2002, 31, 236–241
Raab S., Plate K.H., Different networks, common growth factors: Shared growth factors and receptors of the vascular and the nervous system, Acta Neuropathol., 2007, 113, 607–626
Shen Q., Goderie S.K., Jin L., Karanth N., Sun Y., Abramova N., et al., Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells, Science, 2004, 304, 1338–1340
Sun J., Zhou W., Ma D., Yang Y., Endothelial cells promote neural stem cell proliferation and differentiation associated with VEGF activated Notch and Pten signaling, Dev. Dyn., 2010, 239, 2345–2353
Shen Q., Wang Y., Kokovay E., Lin G., Chuang S.M., Goderie S.K., et al., Adult SVZ stem cells lie in a vascular niche: A quantitative analysis of niche cell-cell interactions, Cell Stem Cell, 2008, 3, 289–300
Ye H., Liu J., Wu J.Y., Cell adhesion molecules and their involvement in autism spectrum disorder, Neurosignals, 2011, 18, 62–71
Jamain S., Quach H., Betancur C., Råstam M., Colineaux C., Gillberg I.C., et al., Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism, Nat. Genet., 2003, 34, 27–29
Laumonnier F., Bonnet-Brilhault F., Gomot M., Blanc R., David A., Moizard M.P., et al., X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family, Am. J. Hum. Genet., 2004, 74, 552–557
Feng J., Schroer R., Yan J., Song W., Yang C., Bockholt A, et al., High frequency of neurexin 1beta signal peptide structural variants in patients with autism, Neurosci. Lett., 2006, 409, 10–13
Kim H.G., Kishikawa S., Higgins A.W., Seong I.S., Donovan D.J., Shen Y., et al., Disruption of neurexin 1 associated with autism spectrum disorder, Am. J. Hum. Genet., 2008, 82, 199–207
Chen S.X., Tari P.K., She K., Haas K., Neurexin-neuroligin cell adhesion complexes contribute to synaptotropic dendritogenesis via growth stabilization mechanisms in vivo, Neuron, 2010, 67, 967–983
Chih B., Engelman H., Scheiffele P., Control of excitatory and inhibitory synapse formation by neuroligins, Science, 2005, 307, 1324–1328
Budreck E.C., Scheiffele P., Neuroligin-3 is a neuronal adhesion protein at GABAergic and glutamatergic synapses, Eur. J. Neurosci., 2007, 26, 1738–1748
Hirao K., Hata Y., Ide N., Takeuchi M., Irie M., Yao I., et al., A novel multiple PDZ domain-containing molecule interacting with N-methyl-D-aspartate receptors and neuronal cell adhesion proteins, J. Biol. Chem., 1998, 273, 21105–21110
Barrow S.L., Constable J.R., Clark E., El-Sabeawy F., McAllister A.K., Washbourne P., Neuroligin1: A cell adhesion molecule that recruits PSD-95 and NMDA receptors by distinct mechanisms during synaptogenesis, Neural Dev., 2009, 4, 17
Murase S., Mosser E., Schuman E.M., Depolarization drives betacatenin into neuronal spines promoting changes in synaptic structure and function, Neuron, 2002, 35, 91–105
Stan A., Pielarski K.N., Brigadski T., Wittenmayer N., Fedorchenko O., Gohla A., et al., Essential cooperation of N-cadherin and neuroligin-1 in the transsynaptic control of vesicle accumulation, Proc. Natl. Acad. Sci. U.S.A., 2010, 107, 11116–11121
Yu X., Malenka R.C., β-catenin is critical for dendritic morphogenesis, Nat. Neurosci., 2003, 6, 1169–1177
Abe K., Takeichi M., NMDA-receptor activation induces calpainmediated β-catenin cleavages for triggering gene expression, Neuron, 2007, 53, 387–397
Derksen M.J., Ward N.L., Hartle K.D., Ivanco T.L., MAP2 and synaptophysin protein expression following motor learning suggests dynamic regulation and distinct alterations coinciding with synaptogenesis, Neurobiol. Learn. Mem., 2007, 87, 404–415
Antar L.N., Afroz R., Dictenberg J.B., Carroll R.C., Bassell G.J., Metabotropic glutamate receptor activation regulates fragile X mental retardation protein and Fmr1 mRNA localization differentially in dendrites and at synapses, J. Neurosci., 2004, 24, 2648–2655
Wang H., Dictenberg J.B., Ku L., Li W., Bassell G.J., Feng Y., Dynamic association of the fragile X mental retardation protein as a messenger ribonucleoprotein between microtubules and polyribosomes, Mol. Biol. Cell, 2008, 19, 105–114
Nimchinsky E.A., Oberlander A.M., Svoboda K., Abnormal development of dendritic spines in FMR1 knock-out mice, J. Neurosci., 2001, 21, 5139–5146
Allin E.F., Evolution of the mammalian middle ear, J. Morphol., 1975, 147, 403–437
Sakarya O., Armstrong K.A., Adamska M., Adamski M., Wang I.F., Tidor B., et al., A post-synaptic scaffold at the origin of the animal kingdom, PLoS ONE, 2007, 2, e506
Nickel M., Evolutionary emergence of synaptic nervous systems: What can we learn from the non-synaptic, nerveless Porifera? Invertebr. Biol., 2010, 129, 1–16
Pinto D., Pagnamenta A.T., Klei L., Anney R., Merico D., Regan R., et al., Functional impact of global rare copy number variation in autism spectrum disorders, Nature, 2010, 466, 368–372
Kolkova K., Novitskaya V., Pedersen N., Berezin V., Bock E., Neural cell adhesion molecule-stimulated neurite outgrowth depends on activation of protein kinase C and the Ras-mitogen-activated protein kinase pathway, J. Neurosci., 2000, 20, 2238–2246
Chang L., Karin M., Mammalian MAP kinase signalling cascades, Nature, 2001, 410, 37–40
Laws S.C., Carey S.A., Ferrell J.M., Bodman G.J., Cooper R.L., Estrogenic activity of octylphenol, nonylphenol, bisphenol A and methoxychlor in rats, Toxicol. Sci., 2000, 54, 154–167
Hertz-Picciotto I., Delwiche L., The rise in autism and the role of age at diagnosis, Epidemiology, 2009, 20, 84–90
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Williams, E.L., Casanova, M.F. Above genetics: Lessons from cerebral development in autism. Translat.Neurosci. 2, 106–120 (2011). https://doi.org/10.2478/s13380-011-0016-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s13380-011-0016-3