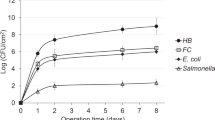
Abstract
Understanding the factors influencing the transport of microbial pathogens, such as Salmonella and Escherichia coli, through porous media is critical to protecting drinking water supplies. The production of biofilms, along with individual biofilm-associated components, such as tafi, is believed to hinder transport of microorganisms through soil. This study investigated the relationship between biofilm formation and tafi production and the transport of environmental Salmonella through porous media. Thirty-two Salmonella isolates were initially assayed for their ability to form biofilms, from which a subset of these was selected to represent a range of high and low biofilm-formation potential and tafi formation capabilities. These were subsequently examined in unsaturated sand columns for transport characteristics. No obvious correlation was observed between Salmonella phenotypes and column retention. The results indicated that while transport of well-characterized laboratory E. coli strains can often be hindered by the presence of tafi and the potential to form biofilms, the presence of tafi did not retard the transport of the Salmonella strains.
Similar content being viewed by others
References
Abu-Lail N.I. & Camesano T.A. 2003. Role of lipopolysaccharides in the adhesion, retention, and transport of Escherichia coli JM109. Environ. Sci. Technol. 37: 2173–2183.
Bjergbaek L.A. & Roslev P. 2005. Formation of nonculturable Escherichia coli in drinking water. J. Appl. Microbiol. 99: 1090–1098.
Brombacher E. et al. 2003. The curli biosynthesis regulator CsgD co-ordinates the expression of both positive and negative determinants for biofilm formation in Escherichia coli. Microbiology 149: 2847–2857.
Da Re S. & Gingo J.-M. 2006. A CsgD-Independent Pathway for cellulose production and biofilm formation in Escherichia coli. J. Bacteriol. 188: 3073–3087.
Gebbink M.F.B.G. et al. 2005. Amyloids — a functional coat for microorganisms. Nat. Rev. Microbiol. 3: 333–341.
Ginn T.R. et al. 2002. Processes in microbial transport in the natural subsurface. Adv. Water Res. 25: 1017–1042.
Gualdi L. et al. Cellulose modulates biofilm formation by counteracting curli-mediated colonization of solid surfaces in Escherichia coli. Microbiology 154: 2017–2024.
Hall-Stoodley L. at al. 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2: 95–108.
Hammar M. et al. 1996. Nucleator-dependent intercellular assembly of adhesive tafi organelles in Escherichia coli. P. Natl. Acad. Sci. USA 93: 6562–6566.
Heaton J.C. & Jones K. 2008. Microbial contamination of fruit and vegetables and the behavior of enteropathogens in the phyllosphere: a review. J. Appl. Microbiol. 104: 613–626.
Jonas K. et al. 2007. Roles of curli, cellulose and BapA in Salmonella biofilm morphology studied by atomic force microscopy. BMC Microbiol. 7: 70.
Kaneene J.B. et al. 2008. Changes in tetracycline susceptibility of enteric bacteria following switching to nonmedicated milk replacer for dairy calves. J. Clin. Microbiol. 46: 1968–1967.
Landini P. & Zehnder A.J. 2002. The global regulatory hns gene negatively affects adhesion to solid surfaces by anaerobically grown Escherichia coli by modulating the expression of lipopolysaccharide and flagellar genes. J. Bacteriol. 184: 1522–1529.
Macler B.A. & Merkle J.C. 2000. Current knowledge on groundwater microbial pathogens and their control. Hydrogeol. J. 8: 29–40.
Olsén A. et al. 1993. Environmental regulation of curli production in Escherichia coli. Infect. Agent. Dis. 2: 272–274.
Prouty A.M. et al. 2002. Biofilm formation and interaction with the surfaces of gallstone by Salmonella spp. Infect. Immun. 70: 2640–2649.
Reisner A. et al. 2006. In vitro biofilm formation of commensal and pathogenic Escherichia coli strains: impact of environmental and genetic factors. J. Bacteriol. 188: 3572–3581.
Rijnaarts H.H.M. et al. 1995. Reversibility and mechanism of bacterial adhesion. Colloid Surface B. 4: 5–22.
Römling U. et al. 1998. Curli fibers are highly conserved between Salmonella typhimurium and Escherichia coli with respect to operon structure and regulation. J. Bacteriol. 180: 722–731.
Sambrook J. et al. 1989. Molecular Cloning: A Laboratory Manual, Vol. 3, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Stevik T.K. et al. 2004. Retention and removal of pathogenic bacteria in wastewater percolating through porous media: a review. Water Res. 38: 1355–1367.
Toba, F.A. 2008 Characterization of the physiological implications of Defective Lambdoid Phage DLP12 in Escherichia coli. PhD Dissertation, Cornell University
Tufenkji N. 2007. Modeling microbial transport in porous media: Traditional approaches and recent developments. Adv. Water Resour. 30: 1455–1469.
Vidal O. et al. 1998. Isolation of an Escherichia coli K-12 mutant strain able to form biofilms on inert surfaces: involvement of a new ompR allele that increases tafi expression. J. Bacteriol. 180: 2442–2491.
Walker S.L. et al. 2005. Influence of growth phase on adhesion kinetics of Escherichia coli D21g. Appl. Environ. Microb. 71: 3093–3099.
Winfield M.D. & Groisman E.A. 2003. Role of nonhost environments in the lifestyles of Salmonella and Escherichia coli. Appl. Environ. Microb. 69: 3687–3694.
Yang H.H. et al. 2004. High diversity among environmental Escherichia coli isolates from a bovine feedlot. Appl. Environ. Microb. 70: 1528–1536.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Salvucci, A.E., Zhang, W., Morales, V.L. et al. The impact of biofilm-forming potential and tafi production on transport of environmental Salmonella through unsaturated porous media. Biologia 64, 460–464 (2009). https://doi.org/10.2478/s11756-009-0102-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-009-0102-y