Abstract
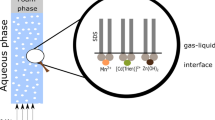
In the paper, simultaneous removal of Al(III) and Cu(II) from dilute aqueous solutions by ion and precipitate flotation methods is investigated. Influence of the pH of the initial solution, the surface active collector concentration and the gas flow rate on the final removal ratio and the course of ion and precipitate flotations is presented. The results show that simultaneous flotations of Al(OH)3 and Cu(OH)2 insoluble species occur allowing to achieve their almost complete removal in the pH range between 7 and 9. An increase of the surface active agent concentration causes a decrease of the final removal ratio as well as of the flotation rate constant. An increase of the gas flow rate results in an increase of ion and precipitate flotation rates.
Similar content being viewed by others
References
Bartkiewicz, B. (2007). Industrial wastewaters treatment. Warsaw, Poland: PWN. (in Polish)
Blais J. F., Djedidi, Z., Ben Cheikh, R., Tyagi, R. D., & Mercier, G. (2008). Metals precipitation from effluents: Review. Practice Periodical of Hazardous, Toxic, and Radioactive Waste Management, 12, 135–149. DOI: 10.1061/(ASCE)1090-025x(2008)12:3(135).
Charewicz, W. A., Hołowiecka, B. A.,& Walkowiak, W. (1999). Selective flotation of zinc(II) and silver(I) ions from dilute aqueous solutions. Separation Science and Technology, 34, 2447–2460. DOI: 10.1081/ss-100100784.
Crawford, R. J., Harding, I. H.,& Mainwaring, D. E. (1993). Adsorption and coprecipitation of single heavy metal ions onto the hydrated oxides of iron and chromium. Langmuir, 9, 3050–3056. DOI: 10.1021/la00035a051.
Degen, A.,& Kosec, M. (2000). Effect of pH and impurities on the surface charge of zinc oxide in aqueous solution. Journal of the European Ceramic Society, 20, 667–673. DOI: 10.1016/s0955-2219(99)00203-4.
Ehrampoush, M. H., Salmani, M. H., Ghaneian, M. T., Davoudi, M.,& Fallahzadeh, M. H. (2011). Selectivity in removal of cadmium (II) from mixed metal effluents using ion flotation. World Applied Sciences Journal, 13(1), 52–59.
Filippov, L. O. (2000). Ion flotation. In M. Cooke, & C. F. Poole (Eds.), Encyclopedia of separation science (Level III, pp. 3179–3186). San Diego, CA, USA: Academic Press. DOI: 10.1016/bo-12-226770-2/05811-7.
Fu, F. L., & Wang, Q. (2011). Removal of heavy metal ions from wastewaters: A review. Journal of Environmental Management, 92, 407–418. DOI:10.1016/j.jenvman.2010.11.011.
Ghazy, S. E.,& El-Morsy, S. M. (2008). Precipitate flotation of aluminum and copper. Trends in Applied Sciences Research, 3, 14–24. DOI:10.3923/tasr.2008.14.24.
Grieves, R. B.,& Bhattacharyya, D. (1967). The foam separation of colloidal ferric oxide with an anionic and a cationic surfactant. Journal of the American Oil Chemists’ Society, 44, 498–501. DOI: 10.1007/bf02908546.
Huang, S. D., Tzuoo, J. J., Gau, J. Y., Hsieh, H. S.,& Fann, C. F. (1984). Effect of Al(III) as an activator for adsorbing colloid flotation. Separation Science and Technology, 19, 1061–1072. DOI: 10.1080/01496398408058348.
Ibezim-Ezeani, M. U., Okoye, F. A.,& Akaranta, O. (2012). Kinetic studiem on the removal of some metal ions from aqueous solution using modified Orange mesocarp extract. International Journal of Water Resources and Environmental Engineering, 4, 192–200. DOI: 10.5897/IJWREE.11.088.
Jensen, W. B. (2012). The quantification of electronegativity: Some precursors. Journal of Chemical Education, 89, 94–96. DOI:10.1021/ed1011822.
Jurkiewicz, K. (1984). Studies on the separation of cadmium from solutions by foam separation. II. Precipitate flotation of cadmium hydroxide. Separation Science and Technology, 19, 1051–1060. DOI: 10.1080/01496398408058347.
Jurkiewicz, K. (2005). Adsorptive bubble separation of zinc and cadmium cations in the presence of ferric and aluminum hydroxides. Journal of Colloid and Interface Science, 286, 559–563. DOI:10.1016/j.jcis.2005.01.061.
Kawalec-Pietrenko, B.,& Selecki, A. (1984). Investigations of kinetics of removal of trivalent chromium salts from aqueous solutions using ion and precipitate flotation methods. Separation Science and Technology, 19, 1025–1038. DOI: 10.1080/01496398408058345.
Kurniawan, T. A., Chan, G. Y. S., Lo, W. H.,& Babel, S. (2006). Physico-chemical treatment techniques for wastewater laden with heavy metals. Chemical Engineering Journal, 118, 83–98. DOI:10.1016/j.cej.2006.01.015.
Leja, J. (1982). Surface chemistry of froth flotation. New York, NY, USA: Plenum Press.
Marczenko, Z., & Balcerzak, M. (1998). Copper. In Spectrophotometrical methods in inorganic analysis (pp. 284–296). Warsaw, Poland: PWN. (in Polish)
Medina, B. Y., Torem, M. L.,& de Mesquita, L. M. S. (2005). On the kinetics of precipitate flotation of Cr III using sodium dodecylsulfate and ethanol. Minerals Engineering, 18, 225–231. DOI:10.1016/j.mineng.2004.08.018.
Mochizuki, T.,& Kuroda, R. (1982). Rapid continuous determination of aluminum in copper-base alloys by flow-injection spectrophotometry. Fresenius’ Zeitschrift für Analytische Chemie, 311, 11–15. DOI: 10.1007/bf00493492.
Parks, G. A. (1965). The isoelectric points of solid oxides, solid hydroxides, and aqueous hydroxo complex systems. Chemical Reviews, 65(2), 177–198. DOI: 10.1021/cr60234a002.
Puigdomenech, I. (2010). MEDUSA [computer software]. Stockholm, Sweden: Royal Institute of Technology.
Reyes, M., Patino, F., Escudero, R., Pérez, M., Flores, M. U.,& Reyes, I. A. (2012). Kinetics and hydrodynamics of silver ion flotation. Journal of the Mexican Chemical Society, 56(4), 408–416.
Rubin, A. J., Johnson, J. D.,& Lamb, J. C., III (1966). Comparison of variables in ion and precipitate flotation. Industrial & Engineering Chemistry Process Design and Developments, 5, 368–375. DOI: 10.1021/i260020a004.
Rubin, A. J.,& Johnson, J. D. (1967). Effect of pH on ion and precipitate flotation systems. Analytical Chemistry, 39, 298–302. DOI: 10.1021/ac60247a009.
Shakir, K.,& Samy, S. (1979). Kinetic studies on the foam separation of thorium (IV) with sodium lauryl sulphate. Colloid and Polymer Science, 257, 420–426. DOI: 10.1007/bf01521579.
Stoica, L., Oproiu, G. C., Cosmeleata, R.,& Dinculescu, M. (2003). Kinetics of Cu2+ separation by flotation. Separation Science and Technology, 38, 613–632.DOI: 10.1081/ss-120016654.
Uribe-Salas, A., Pérez-Garibay, R., Nava-Alonso, F.,& Castro-Román, M. (2005). A kinetic model for Pb2+ flotation with sodium dodecylsulfate in a batch column. Separation Science and Technology, 40, 3225–3237. DOI: 10.1080/01496390500385426.
Walkowiak, W. (1991). Mechanism of selective ion flotation. 1. Selective flotation of transition metal cations. Separation Science and Technology, 26, 559–568. DOI: 10.1080/01496499108050490.
Wömmel, S.,& Calmano, W. (1992). Studies on separation of heavy metals from acidic solutions by foam fractionation with respect to an application on acidic soil extracts. Acta Hydrochimica et Hydrobiologica, 20, 265–268.
Zhang, J., Jing, Y. J., Wu, Z. L.,& Li, Q. (2009). Removal of trace Cu2+ from aqueous solution by foam fractionation. Desalination, 249, 503–506. DOI:10.1016/j.desal.2008.07.028.
Zouboulis, A. I.,& Matis, K. A. (1987). Ion flotation in environmental technology. Chemosphere, 16, 623–631. DOI: 10.1016/0045-6535(87)90275-x.
Zouboulis, A. I. (1995). Silver recovery from aqueous streams using ion flotation. Minerals Engineering, 8, 1477–1488. DOI: 10.1016/0892-6875(95)00112-3.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kawalec-Pietrenko, B., Rybarczyk, P. Al(III) and Cu(II) simultaneous foam separation: Physicochemical problems. Chem. Pap. 68, 890–898 (2014). https://doi.org/10.2478/s11696-014-0534-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-014-0534-x