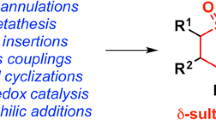
Abstract
The stereoselective synthesis of sulfamisterin I and its unnatural analogues II and V in their protected form was achieved through a common strategy. The Wittig reaction of aldehydes VIII and IX with the C14 hydrophobic side-chain X served as the key C-C connecting transformation. Subsequent functional group inter-conversions in the coupling products XI and XX completed the total synthesis.
Similar content being viewed by others
References
Azuma, H., Tamagaki, S., & Ogino, K. (2000). Stereospecific total syntheses of sphingosine and its analogues from l-serine. The Journal of Organic Chemistry, 65, 3538–3541. DOI: 10.1021/jo991447x.
Byun, H. S., Lu, X., & Bittman, R. (2006). Stereoselective total synthesis of serine palmitoyl-CoA transferase inhibitors. Synthesis, 2006, 2447–2474. DOI: 10.1055/s-2006-942475.
Gonda, J., Martinková, M., Raschmanová, J., & Balentová, E. (2006). Creation of quaternary stereocentres via [3,3]-sigmatropic rearrangement of allylic thiocyanates. A synthetic approach to (+)-myriocin. Tetrahedron: Asymmetry, 17, 1875–1882. DOI: 10.1016/j.tetasy.2006.06.032.
Hanada, K. (2003). Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochimica et Biophysica Acta, 1632, 16–30. DOI: 10.1016/s1388-1981(03)00059-3.
Hansen, M. M., Harkness, A. R., Coffey, D. S., Bordwell, F. G., & Zhao, Y. (1995). Substrate acidities and conversion times for reactions of amides with di-tert-butyl dicarbonate. Tetrahedron Letters, 36, 8949–8952. DOI: 10.1016/0040-4039(95)01931-7.
Kang, S. H., Kang, S. Y., Lee, H. S., & Buglass, A. J. (2005). Total synthesis of natural tert-alkylamino hydroxy carboxylic acids. Chemical Reviews, 105, 4537–4558. DOI: 10.1021/cr040608g.
Liav, A., & Goren, M. B. (1984). Sulfate as a blocking group in alkali-catalyzed permethylation: an alternative synthesis of 3,4,6-tri-O-methyl-D-glucose. Carbohydrate Research, 131, C8–C10. DOI: 10.1016/0008-6215(84)85419-1.
Martinková, M., Gonda, J., & Raschmanová, J. (2006). Novel furanoid α-substituted α-amino acid as a potent turn mimic in peptide synthesis. Molecules, 11, 564–573. DOI: 10.3390/11070564.
Martinková, M., Gonda, J., Raschmanová, J., & Uhríková, A. (2008). Stereoselective synthesis of both enantiomers of α-(hydroxymethyl)glutamic acid. Tetrahedron: Asymmetry, 19, 1879–1885. DOI: 10.1016/j.tetasy.2008.08.003.
Martinková, M., Gonda, J., Špaková, J., Slaninková, M., & Kuchár, J. (2010). Total synthesis of a protected form of sphingofungin E using the [3,3]-sigmatropic rearrangement of an allylic thiocyanate as the key reaction. Carbohydrate Research, 345, 2427–2437. DOI: 10.1016/j.carres.2010.09.016.
Martinková, M., Gonda, J., Uhríková, A., Špaková, J., & Kuchár, J. (2012a). An efficient synthesis of the polar part of sulfamisterin and its analogs. Carbohydrate Research, 352, 23–36. DOI: 10.1016/j.carres.2012.02.019.
Martinková, M., Gonda, J., Špaková, J., Kuchár, J., & Kožĭšek, J. (2012b). A stereoselective synthesis of an α-substituted α-amino acid as a substructure for the construction of myriocin. Tetrahedron: Asymmetry, 23, 536–546. DOI: 10.1016/j.tetasy.2012.04.012.
Mita, T., Fukuda, N., Roca, F. X., Kanai, M., & Shibasaki, M. (2007). Second generation catalytic asymmetric synthesis of Tamiflu: Allylic substitution route. Organic Letters, 9, 259–262. DOI: 10.1021/ol062663c.
More, J. D., & Finney, N. S. (2002). A simple and advantageous protocol for the oxidation of alcohols with oiodoxybenzoic acid (IBX). Organic Letters, 4, 3001–3003. DOI: 10.1021/ol026427n.
Ohfune, Y., & Shinada, T. (2005). Enatio- and diastereos-elective construction of α,α-disubstituted α-amino acids for the synthesis of biologically active compounds. European Journal of Organic Chemistry, 2005, 5127–5143. DOI: 10.1002/ejoc.200500434.
Payette, D. R., & Just, G. (1981). A total synthesis of the enantiomer of anhydromyriocin (anhydrothermozymocidin). Canadian Journal of Chemistry, 59, 269–282. DOI: 10.1139/v81-044.
Sanders, W. J., Manning, D. D., Koeller, K. M., & Kiessling, L. L. (1997). Synthesis of sulfated trisaccharide ligands for the selectins. Tetrahedron, 53, 16391–16422. DOI: 10.1016/s0040-4020(97)01024-7.
Sano, S., Kobayashi, Y., Kondo, T., Takebayashi, M., Maruyama, S., Fujita, T., & Nagao, Y. (1995). Asymmetric total synthesis of ISP-I (myriocin, thermozymocidin), a potent immunosuppressive principle in the Isaria sinclairii metabolite. Tetrahedron Letters, 36, 2097–2100. DOI: 10.1016/0040-4039(95)00219-3.
Satam, V., Harad, A., Rajule, R., & Pati, H. (2010). 2-Iodoxybenzoic acid (IBX): an efficient hypervalent iodine reagent. Tetrahedron, 66, 7659–7706. DOI: 10.1016/j.tet.2010.07.014.
Sato, H., Maeba, T., Yanase, R., Yamaji-Hasegawa, A., Kobayashi, T., & Chida, N. (2005). Total synthesis and biological activities of (+)-sulfamisterin (AB5366) and its analogues. The Journal of Antibiotics, 58, 37–49. DOI: 10.1038/ja.2005.4.
Shioiri, T., Terao, Y., Irako, N., & Aoyama, T. (1998). Synthesis of topostins B567 and D654 (WB-3559D, flavolipin), DNA topoisomerase I inhibitors of bacterial origin. Tetrahedron, 54, 15701–15710. DOI: 10.1016/s0040-4020(98)00984-3.
Yamaji-Hasegawa, A., Takahashi, A., Tetsuka, Y., Senoh, Y., & Kobayashi, T. (2005). Fungal metabolite sulfamisterin suppresses sphingolipid synthesis through inhibition of serine palmitoyltransferase. Biochemistry, 44, 268–277. DOI: 10.1021/bi048605l.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Špaková Raschmanová, J., Martinková, M., Gonda, J. et al. Stereoselective total synthesis of protected sulfamisterin and its analogues. Chem. Pap. 67, 1317–1329 (2013). https://doi.org/10.2478/s11696-013-0392-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11696-013-0392-y