Abstract
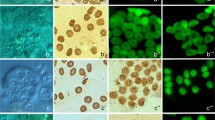
The reproductive organs of both male and female European lobsters (Homarus gammarus) are H-shaped gonads that lie dorsal to the gut on the large hepatopancreas. The ovary consists of a pair of tubular, parallel lobules with a connecting bridge. The germarium of the ovary containing oogonia is concentrated in the center of the ovarian lobe. As oogonesis proceeds, the oocytes move to the peripheral regions of the ovary. The follicle cells begin to surround the oocytes in the previtellogenic stage, and the mature oocytes are completely surrounded by the follicle cells. Carbohydrates exist in both early and late vitellogenic oocytes that give PAS positive reaction. However, their rising protein content in late vitellogenic oocytes makes them stain with Bromophenol blue. Testes show convoluted lobules with a germinal epithelium and a central collecting duct, and the paired vasa deferentia have three distinct parts. Spermatophores are nonpedunculate and tubular, which extrude as a continuous column and consist of a sperm mass covered with primary and secondary layers. The primary layer stains with Bromophenol Blue and gives a PAS positive reaction. But the secondary layer only weakly stains with Bromophenol Blue. The histochemical results may indicate that the function of the two layers is different.
Similar content being viewed by others
References
Prodöhl P.A., Jørstad K.E., Triantafyllidis A., Katsares V., Triantaphyllidis C., Evaluation of genetic impact of aquaculture activities on native populations, A European network, Genimpact, 2006
Holthius L.B., FAO Species Catalogue, Marine lobsters of the World, An annotated and illustrated catalogue of species of interest to fisheries known to date, FAO Fisheries Synopsis, 1991
Schamalenbach I., Buchholz F., Franke H.D., Saborowski R., Improvement of rearing conditions for juvenile lobsters (Homarus gammarus) by coculturing with juvenile isopods (Idotea emarginata), Aquaculture, 2009, 289, 297–303
Ferguson A., Genetic diversity in the European lobster (Homarus gammarus): population structure and impacts of stock enhancement, Fisheries and Agriculture, 2002
Beal B.F., Lobster management in Maine, USA, In: Tully O. (Ed.), The Biology and Management of Clawed Lobster (Homarus gammarus L.) in Europe, Vol.2, Fisheries Resource Series, Bord lascaigh Mhara (Irish Sea Fisheries Board), Dun Lacghaire, Ireland, 2004
Beal B.F., Mercer J.P., O’Conghaile A., Survival and growth of hatchery-reared individuals of the European lobster, Homarus gammarus (L.), in field-based nursery cages on the Irish west coat, Aquaculture, 2002, 210, 137–157
Schmalenbach I., Franke H.D., Potential impact of climate warming on the recruitment of an economically and ecologically important species, the European lobster (Homarus gammarus) at Helgoland, North Sea, Mar. Biol., 2010, 157, 1127–1135
Schmalenbach I., Studies on the developmental conditions of the European lobster (Homarus gammarus Linnaeus, 1758) at the rocky island of Helgoland (German Bight, North Sea), PhD thesis, University of Hamburg, Hamburg, Germany, 2009
Agnalt A.L., Kristiansen T.S., Jorstad K.E., Growth, reproductive cycle, and movement of berried European lobsters (Homarus gammarus) in a local stock off southwestern Norway, J. Mar. Sci., 2006, 64, 288–297
Free E.K., Reproduction in the European Lobster (Homarus gammarus (L.). Proceedings of the Seminar at Kvitsoey 1995: the European lobster Homarus gammarus (L.), Fisk. Havet., 1998, 13, 4–26
Wenner A.M., Page H.M., Siegel P.R., Variation on size at onset of egg production, In: Wenner A.M. (Ed.), Crustacean Issues 3, Factors in Adult Growth, A.A. Balkema, Rotterdam, Boston, 1985
Pearse A.G.E., Histochemistry theoretical and applied, Vol. 2. J. and A. Churchill, London, 1960
Bancroft J.D., Stevens A., Theory and Practice of Histological Techniques, Churchill Livingstone, London, 1982
Talbot P., Helluy S., Reproduction and Embryonic Development, In: Factor J.R. (Ed.), Biology of the Lobster Homarus americanus, Academic Press Inc., San Diego California, 1995
Talbot P., The ovary of the lobster, Homarus americanus I. Architecture of the Mature Ovary, J. Ultra. Mol. Struct. Res., 1981, 76, 249–262
Adiyodi R.G., Subramaniam T., Arthropoda-Crustacea, In: Adiyodi K.G., Adiyodi R.G. (Eds.), Reproductive Biology of Invertebrates I. Oogenesis oviposition and oosorption, Vol. 1, John Wiley and Sons, London, 1983
Krol R.M., Hawkins W.E., Overstreet R.M., Reproductive Components, In: Harrison F.W., Humes A. G. (Eds.), Microscopic Anatomy of Invertebrates, Decapod Crustacea, V. 10., Wiley-Liss, Inc., New York, 1992
Vogt G., Functional Anatomy, In: Holdich D.M. (Ed.), Biology of Freshwater Crayfish, Blackwell Science, USA and Canada, 2002
Hobbs H.Jr, Harvey C., Hobbs H., A comparative study of functional morphology of male reproductive system in the Astacidae with emphasis on the freshwater crayfish (Crustacea, Decapoda), Smithson Institution scholarly Press, Washington, 2007
Howard D.R., Talbot P., In vitro contraction of lobster (Homarus) ovarian muscle: methods for assaying contraction and effects of biogenic amines, J. Exp. Zool., 1992, 263, 356–366
Ando H., Makioka T., Structure of the ovary and mode of oogenesis in a freshwater crayfish, Procambarus clarkii (Girard), Zool. Sci., 1998, 15, 893–901
López Greco L.S., Vazquez F., Rodríguez E.M., Morphology of the male reproductive system and spermatophore formation in the freshwater ‘redclaw’ crayfish Cherax quadricarinatus (Von Martens, 1898) (Decapoda, Parastacidae), Acta Zool., 2007, 88, 223–229
Unis C., Erkan M.B., Morphology and development of the female reproductive system Astacus leptodactylus (Eschscholtz, 1823) (Decapoda, Astacidae), Turkish J. Zool., 2012, 36, 775–784
Erkan M., Tunalı Y., Baş S.S., Male reproductive system morphology and spermatophore formation in Astacus leptodactylus (Eschscholtz, 1823) (Decapoda: Astacidae), J. Crustacean Biol., 2009, 29, 42–50
Vazquez F.J., Tropea C., Lopez Greco L.S., Development of the female reproductive system in the freshwater crayfish Cherax quadricarinatus (Decapoda, Parastacidae), Invertebr. Biol., 2008, 127, 433–443
Kessel R.G., Mechanisms of protein yolk synthesis and deposition in crustacean oocytes, Z. Zellforsch, 1968, 89, 17–38
Farmer A.S.D., Reproduction in Nephrops norvegicus (Decapoda:Nephropidae), J. Zool., 1974, 174, 161–183
Schade M.L., Shivers R.R., Structural modulation of the surface and cytoplasm of oocytes during vitellogenesis in the Lobster, Homarus americanus. An electron microscope-protein tracer study, J. Morphol., 1980, 163, 13–26
Beams H.W., Kessel R.G., Electron microscope studies on developing crayfish oocytes with special reference to the origin of yolk, J. Cell Biol., 1963, 18, 621–649
Abdu U., Yehezkel G., Sagi A., Oocyte development and polypeptide dynamics during ovarian maturation in the red-claw crayfish Cherax quadricarinatus, Invertebr. Reprod. Dev., 2000, 37, 75–83
O’Donovan P., Abraham M., Cohen D., The ovarian cycle during the intermolt in ovigerous Macrobrachium rosenbergi, Aquaculture, 1984, 36, 347–358
Roudy J.C., Amsler M.O., Ovarian development and sexual maturity staging in antarctic krill, Euphausia superba Dana (Euphausiacea), J. Crustacean Biol., 1991, 11, 236–249
Kozak P., Hulak M., Policar T., Tichy F., Studies of annual gonadal development and gonadal ultrastructure in spiny-cheek crayfısh (Orconectes limosus), B. Fr. Peche Piscic., 2007, 384, 15–26
Kulkarni G.K., Glade L., Fingerman M., Oogenesis and effects of neuroendocrine tissues on in vitro synthesis of protein by the ovary of the red swamp crayfish Procambarus clarkii, J. Crustacean Biol., 1991, 11, 513–522
Rudolph E.H., Rojas C.S., Embryonic and early postembryonic development of the burrowing crayfish, Virilastacus araucanius (Faxon, 1914) (Decapoda, Parastacidae) under laboratory conditions, Crustaceana, 2003, 76, 835–850
Beatty S.J., Morgan D.L., Gill H.S., Life history and reproductive biology of the gilgie, Cherax quinquecarinatus a freshwater crayfish endemic to southwestern Australia, J. Crustacean Biol., 2005, 25, 251–262
Rotllant G., Chiva M., Durfort M., Ribes E., Internal anatomy and ultrastructure of the male reproductive system of the Norway lobster Nephrops norvegicus (Decapoda: Astacidea), J. Morph., 2012, 273, 572–585
Rudolph E., New records of intersexuality in the freshwater crayfish Samastacus spinifrons (Decapoda, Parastacidae), J. Crustacean Biol., 2002, 22, 377–389
Noro C., López Greco L.S., Buckup L., Gonad morphology and type of sexuality in Parastacus defossus Faxon 1898, a burrowing, intersexed crayfish from Southern Brazil (Decapoda: Parastacidae), Acta Zool., 2008, 89, 59–67
McLaughlin P.A., Internal anatomy, In: Venberg F.J., Venberg W.B. (Eds.), The Biology of Crustacea, Vol. 5, Academic Press, New York, 1983
Kooda-Cisco M., Talbot P., Ultrastructure and role of the lobster vas deferens in spermatophore formation: the proximal segment, J. Morph., 1986, 188, 91–103
Mann T., Spermatophores, Springer-Verlag, New York, 1984
Aiken D.E., Waddy S.L., Mercer S.M., Confirmation of external fertilization in the American lobster, Homarus americanus, J. Crustacean Biol., 2004, 24, 474–480
Adiyodi K.G., Anilkumar G., Arthropoda-Crustacea, In: Adiyodi A.G., Adiyodi K.G. (Eds.), Reproductive Biology of Invertebrates, Vol. 3 Accessory Sex Glands, Wiley & Sons, Chichester, 1988
Anilkumar G., Sudha K., Subramoniam T., Spermatophore transfer and sperm structure in the Brachyuran carb Metopograpsus messor (Decapoda: Grapsidae), J. Crustacean Biol., 1999, 19, 361–370
Tudge C.C., Comparative ultrastructure of hermit crab spermatozoa (Paguroidea, Anomura, Decapoda), J. Crustacean Biol., 1992, 12, 397–409
Tudge C.C., Spermatophore morphology in the hermit crab families Paguridae and Parapaguridae (Paguroidea, Anomura, Decapoda), Inverteb. Repro. Dev., 1999, 35, 203–214
Kooda-Cisco M.J., Talbot P., A structural analysis of the freshly extruded spermatophore from the lobster, Homarus americanus, J. Morph., 1982, 172, 193–207
Haley S.R., Spermatogenesis and spermatophore production in the Hawaiian red lobster Enoplometopus occidentalis (Randall) (Crustacea, Nephropidae), J. Morph., 1984, 180, 181–193
Hinsch G.W., Walker M.H., The vas deferens of the spider crab, Libinia emarginata, J. Morph., 1974, 143, 1–19
Dudenhausen E.E., Talbot P., An ultrastructural comparison of soft and hardened spermatophores from the crayfish Pacifastacus leniusculus Dana, Can. J. Zool., 1983, 61, 182–194
Beach D., Talbot P., Ultrastructure comparison of sperm from the crayfishes Cherax tenuimanus and Cherax albidus, J. Crustacean Biol., 1987, 7, 205–218
López Greco L.S., Lo Nostro F.L., Structural changes in the spermatophore of the freshwater ‘red claw’ crayfish Cherax quadricarinatus (Von Martens., 1898) (Decapoda, Parastacidae), Acta Zool., 2008, 89, 149–155
Mazia D., Brewer P.A., Alfert M., The cytochemical staining and measurement of protein with mercúric bromophenol blue, Biol. Bull., 1953, 104, 56–67 48
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Erkan, M., Ayun, Y.T. Morphological and histochemical examination of male and female gonads in Homarus gammarus (L. 1758). cent.eur.j.biol. 9, 37–48 (2014). https://doi.org/10.2478/s11535-013-0148-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11535-013-0148-7