Abstract
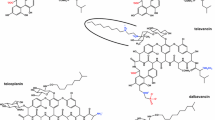
Dalbavancin, oritavancin and telavancin are semisynthetic lipoglycopeptides that demonstrate promise for the treatment of patients with infections caused by multi-drug-resistant Gram-positive pathogens. Each of these agents contains a heptapeptide core, common to all glycopeptides, which enables them to inhibit transglycosylation and transpeptidation (cell wall synthesis). Modifications to the heptapeptide core result in different in vitro activities for the three semisynthetic lipoglycopeptides. All three lipoglycopeptides contain lipophilic side chains, which prolong their half-life, help to anchor the agents to the cell membrane and increase their activity against Gram-positive cocci. In addition to inhibiting cell wall synthesis, telavancin and oritavancin are also able to disrupt bacterial membrane integrity and increase membrane permeability; oritavancin also inhibits RNA synthesis. Enterococci exhibiting the VanA phenotype (resistance to both vancomycin and teicoplanin) are resistant to both dalbavancin and telavancin, while oritavancin retains activity. Dalbavancin, oritavancin and telavancin exhibit activity against VanB vancomycin-resistant enterococci. All three lipoglycopeptides demonstrate potent in vitro activity against Staphylococcus aureus and Staphylococcus epidermidis regardless of their susceptibility to meticillin, as well as Streptococcus spp. Both dalbavancin and telavancin are active against vancomycin-intermediate S. aureus (VISA), but display poor activity versus vancomycin-resistant S. aureus (VRSA). Oritavancin is active against both VISA and VRSA. Telavancin displays greater activity against Clostridium spp. than dalbavancin, oritavancin or vancomycin.
The half-life of dalbavancin ranges from 147 to 258 hours, which allows for once-weekly dosing, the half-life of oritavancin of 393 hours may allow for one dose per treatment course, while telavancin requires daily administration. Dalbavancin and telavancin exhibit concentration-dependent activity and AUC/MIC (area under the concentration-time curve to minimum inhibitory concentration ratio) is the pharmacodynamic parameter that best describes their activities. Oritavancin’s activity is also considered concentration-dependent in vitro, while in vivo its activity has been described by both concentration and time-dependent models; however, AUC/MIC is the pharmacodynamic parameter that best describes its activity.
Clinical trials involving patients with complicated skin and skin structure infections (cSSSIs) have demonstrated that all three agents are as efficacious as comparators. The most common adverse effects reported with dalbavancin use included nausea, diarrhoea and constipation, while injection site reactions, fever and diarrhoea were commonly observed with oritavancin therapy. Patients administered telavancin frequently reported nausea, taste disturbance and insomnia. To date, no drug-drug interactions have been identified for dalbavancin, oritavancin or telavancin. All three of these agents are promising alternatives for the treatment of cSSSIs in cases where more economical options such as vancomycin have been ineffective, in cases of reduced vancomycin susceptibility or resistance, or where vancomycin use has been associated with adverse events.






Similar content being viewed by others
References
Stryjewski ME, Chambers HF. Skin and soft-tissue infections caused by community-acquired methicillin-resistant Staphylococcus aureus. Clin Infect Dis 2008 Jun; 46 Suppl. 5: S368–77
Zhanel GG, Trapp S, Gin AS, et al. Dalbavancin and telavancin: novel lipoglycopeptides for the treatment of Gram-positive infections. Expert Rev Anti Infect Ther 2008 Feb; 6(1): 67–81
Bailey J, Summers KM. Dalbavancin: a new lipoglycopeptide antibiotic. Am J Health Syst Pharm 2008 Apr; 65(7): 599–610
Charneski L, Patel PN, Sym D. Telavancin: a novel lipoglycopeptide antibiotic. Ann Pharmacother 2009 May; 43(5): 928–38
Bhavnani SM, Ballow CH. New agents for Gram-positive bacteria. Curr Opin Microbiol 2000 Oct; 3(5): 528–34
Drew RH. Emerging options for treatment of invasive, multidrug-resistant Staphylococcus aureus infections. Pharmacotherapy 2007 Feb; 27(2): 227–49
Chen AY, Zervos MJ, Vazquez JA. Dalbavancin: a novel antimicrobial. Int J Clinic Practice 2007 May; 61(5): 853–63
Attwood RJ, LaPlante KL. Telavancin: a novel lipoglycopeptide antimicrobial agent. Am J Health Syst Pharm 2007 Nov; 64(22): 2335–48
Guay DR. Oritavancin and tigecycline: investigational antimicrobials for multidrug-resistant bacteria. Pharmacotherapy 2004 Jan; 24(1): 58–68
Cornaglia G, Rossolini GM. Forthcoming therapeutic perspectives for infections due to multidrug-resistant Gram-positive pathogens. Clin Microbiol Infect 2009 Mar; 15(3): 218–23
Allen NE, LeTourneau DL, Hobbs Jr JN, et al. Hexa-peptide derivatives of glycopeptide antibiotics: tools for mechanism of action studies. Antimicrob Agents Chemother 2002 Aug; 46(8): 2344–8
Sosio M, Stinchi S, Beltrametti F, et al. The gene cluster for the biosynthesis of the glycopeptide antibiotic A40926 by nonomuraea species. Chem Biol 2003 Jun; 10(6): 541–9
Billeter M, Zervos MJ, Chen AY, et al. Dalbavancin: a novel once-weekly lipoglycopeptide antibiotic. Clin Infect Dis 2008 Feb;46(4): 577–83
Kim A, Kuti JL, Nicolau DP. Review of dalbavancin, a novel semisynthetic lipoglycopeptide. Expert Opin Investig Drugs 2007 May; 16(5): 717–33
Malabarba A, Goldstein BP. Origin, structure, and activity in vitro and in vivo of dalbavancin. J Antimicrob Chemother 2005 Mar; 55 Suppl. 2: ii15–20
Allen NE, Nicas TI. Mechanism of action of oritavancin and related glycopeptide antibiotics. FEMS Microbiol Rev 2003 Jan; 26(5): 511–32
Cooper RD, Snyder NJ, Zweifel MJ, et al. Reductive alkylation of glycopeptide antibiotics: synthesis and antibacterial activity. J Antibiot (Tokyo) 1996 Jun; 49(6): 575–81
Mercier RC, Hrebickova L. Oritavancin: a new avenue for resistant Gram-positive bacteria. Expert Rev Anti Infect Therapy 2005 Jun; 3(3): 325–32
Higgins DL, Chang R, Debabov DV, et al. Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2005 Mar; 49(3): 1127–34
Laohavaleeson S, Kuti JL, Nicolau DP. Telavancin: a novel lipoglycopeptide for serious gram-positive infections. Expert Opin Investig Drugs 2007 Mar; 16(3): 347–57
Reynolds PE. Structure biochemistry and mechanism of action of glycopeptide antibiotics. Eur J Clin Microbiol Infect Dis 1989; 8(11): 943–50
Belley A, Neesham-Grenon E, McKay G, et al. Oritavancin kills stationary-phase and biofilm Staphylococcus aureus cells in vitro. Antimicrob Agents Chemother 2009 Mar; 53(3): 918–25
Patti GJ, Kim SJ, Yu TY, et al. Vancomycin and oritavancin have different modes of action in Enterococcus faecium. J Mol Biol 2009 Oct 9; 392(5): 1178–91
Kim SJ, Cegelski L, Stueber D, et al. Oritavancin exhibits dual mode of action to inhibit cell-wall biosynthesis in Staphylococcus aureus. J Mol Biol 2008 Mar 14; 377(1): 281–93
Belley A, Harris R, Beveridge T, et al. Ultrastructural effects of oritavancin on methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. Antimicrob Agents Chemother 2009 Feb; 53(2): 800–4
Arthur M, Depardieu F, Reynolds P, et al. Moderate-level resistance to glycopeptide LY333328 mediated by genes of the vanA and vanB clusters in enterococci. Antimicrob Agents Chemother 1999 Aug; 43(8): 1875–80
Courvalin P. Vancomycin resistance in gram-positive cocci. Clin Infect Dis 2006 Jan 1; 42 Suppl. 1: S25–34
Zhanel GG, DeCorby M, Nichol KA, et al. Antimicrobial susceptibility of 3931 organisms isolated from intensive care units in Canada: Canadian National Intensive Care Unit Study, 2005/2006. Diagnostic Microb Infect Dis 2008 Sep; 62(1): 67–80
Biedenbach DJ, Bell JM, Sader HS, et al. Activities of dalbavancin against a worldwide collection of 81,673 gram-positive bacterial isolates. Antimicrob Agents Chemother 2009 Mar; 53(3): 1260–3
Biedenbach DJ, Ross JE, Fritsche TR, et al. Activity of dalbavancin tested against Staphylococcus spp. and beta-hemolytic Streptococcus spp. isolated from 52 geographically diverse medical centers in the United States. J Clinic Microb 2007 Mar; 45(3): 998–1004
Goldstein BP, Draghi DC, Sheehan DJ, et al. Bactericidal activity and resistance development profiling of dalbavancin. Antimicrob Agents Chemother 2007 Apr; 51(4): 1150–4
Jones RN, Stilwell MG, Sader HS, et al. Spectrum and potency of dalbavancin tested against 3322 Gram-positive cocci isolated in the United States Surveillance Program (2004). Diagnostic Microb Infect Dis 2006 Feb; 54(2): 149–53
Gales AC, Sader HS, Jones RN. Antimicrobial activity of dalbavancin tested against Gram-positive clinical isolates from Latin American medical centres. Clin Microbiol Infect 2005 Feb; 11(2): 95–100
Arhin FF, Drahi DC, Pillar CM, et al. Comparative in vitro activity profile of oritavancin against recent gram-positive clinical isolates. Antimicrob Agents Chemother 2009 Nov; 53(11): 4762–71
Johnson DM, Fritsche TR, Sader HS, et al. Evaluation of dalbavancin in combination with nine antimicrobial agents to detect enhanced or antagonistic interactions. Int J Antimicrob Agents 2006 Jun; 27(6): 557–60
Boylan CJ, Campanale K, Iversen PW, et al. Pharmaco-dynamics of oritavancin (LY333328) in a neutropenic-mouse thigh model of Staphylococcus aureus infection. Antimicrob Agents Chemother 2003 May; 47(5): 1700–6
Lefort A, Saleh-Mghir A, Garry L, et al. Activity of LY333328 combined with gentamicin in vitro and in rabbit experimental endocarditis due to vancomycin-susceptible or -resistant Enterococcus faecalis. Antimicrob Agents Chemother 2000 Nov; 44(11): 3017–21
Bhavnani SM, Passarell JA, Owen JS, et al. Pharmacokinetic-pharmacodynamic relationships describing the efficacy of oritavancin in patients with Staphylococcus aureus bacteremia. Antimicrob Agents Chemother 2006 Mar; 50(3): 994–1000
Baltch AL, Smith RP, Ritz WJ, et al. Comparison of inhibitory and bactericidal activities and postantibiotic effects of LY333328 and ampicillin used singly and in combination against vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother 1998 Oct; 42(10): 2564–8
Noviello S, Ianniello F, Esposito S. In vitro activity of LY333328 (oritavancin) against Gram-positive aerobic cocci and synergy with ciprofloxacin against enterococci. J Antimicrob Chemother 2001 Aug; 48(2): 283–6
Pankuch GA, Appelbaum PC. Postantibiotic effects of telavancin against 16 gram-positive organisms. Antimicrob Agents Chemother 2009 Mar; 53(3): 1275–7
Hegde SS, Reyes N, Wiens T, et al. Pharmacodynamics of telavancin (TD-6424), a novel bactericidal agent, against gram-positive bacteria. Antimicrob Agents Chemother 2004 Aug; 48(8): 3043–50
Van Bambeke F, Van Laethem Y, Courvalin P, et al. Glycopeptide antibiotics: from conventional molecules to new derivatives. Drugs 2004; 64(9): 913–36
Seltzer E, Dorr MB, Goldstein BP, et al. Once-weekly dalbavancin versus standard-of-care antimicrobial regimens for treatment of skin and soft-tissue infections. Clin Infect Dis 2003 Nov; 37(10): 1298–303
Raad I, Darouiche R, Vazquez J, et al. Efficacy and safety of weekly dalbavancin therapy for catheter-related bloodstream infection caused by gram-positive pathogens. Clin Infect Dis 2005 Feb; 40(3): 374–80
Jauregui LE, Babazadeh S, Seltzer E, et al. Randomized, double-blind comparison of once-weekly dalbavancin versus twice-daily linezolid therapy for the treatment of complicated skin and skin structure infections. Clin Infect Dis 2005 Nov; 41(10): 1407–15
Stryjewski ME, O’Riordan WD, Lau WK, et al. Telavancin versus standard therapy for treatment of complicated skin and soft-tissue infections due to gram-positive bacteria. Clin Infect Dis 2005 Jun; 40(11): 1601–7
Stryjewski ME, Chu VH, O’Riordan WD, et al. Telavancin versus standard therapy for treatment of complicated skin and skin structure infections caused by gram-positive bacteria: FAST 2 study. Antimicrob Agents Chemother 2006 Mar; 50(3): 862–7
Hanaki H, Kuwahara-Arai K, Boyle-Vavra S, et al. Activated cell-wall synthesis is associated with vancomycin resistance in methicillin-resistant Staphylococcus aureus clinical strains Mu3 and Mu50. J Antimicrob Chemother 1998; 42(2): 199–209
Kosowska-Shick K, Clark C, Pankuch GA, et al. Activity of telavancin against staphylococci and enterococci determined by MIC and resistance selection studies. Antimicrob Agents Chemother 2009 Oct; 53(10): 4217–24
Arhin FF, Sarmiento I, Belley A, et al. Effect of polysorbate 80 on oritavancin binding to plastic surfaces: implications for susceptibility testing. Antimicrob Agents Chemother 2008 May; 52(5): 1597–603
Arhin FF, Sarmiento I, Parr TR, et al. Comparative in vitro activity of oritavancin against Staphylococcus aureus strains that are resistant intermediate or heteroresistant to vancomycin. J Antimicrob Chemother 2009 Oct; 64(4): 868–70
Vashisht R, Tailor F, Langner S, et al. Activity of oritavancin against Staphylococcus aureus, S. epidermidis, Enterococcus spp. and Streptococcus pneumoniae, isolated from Canadian hospitals: results of CANWARD 2007 [abstract no. C1-146]. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); 2008 Oct 25-28; Washington, DC
Anderson DL. Oritavancin for skin infections. Drugs Today (Barc) 2008 Aug; 44(8): 563–75
Hellmark B, Unemo M, Nilsdotter-Augustinsson A, et al. Antibiotic susceptibility among Staphylococcus epidermidis isolated from prosthetic joint infections with special focus on rifampicin and variability of the rpoB gene. Clin Microbiol Infect 2009 Mar; 15(3): 238–44
Badal R, Hoban D, Johnson A, et al. Oritavancin comparative in vitro activity against hospital-acquired enterococci from multicenter surveillance: the ORION study [abstract]. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2008 Oct 25-28; Washington, DC
Jansen WT, Verel A, Verhoef J, et al. In vitro activity of telavancin against Gram-positive clinical isolates recently obtained in Europe. Antimicrob Agents Chemother 2007 Sep; 51(9): 3420–4
Draghi DC, Benton BM, Krause KM, et al. In vitro activity of telavancin against recent Gram-positive clinical isolates: results of the 2004-05 Prospective European Surveillance Initiative. J Antimicrob Chemother 2008 Jul; 62(1): 116–21
Draghi DC, Benton BM, Krause KM, et al. Comparative surveillance study of telavancin activity against recently collected Gram-positive clinical isolates from across the United States. Antimicrob Agents Chemother 2008 Jul; 52(7): 2383–8
Krause KM, Barriere SL, Kitt MM, et al. Activity of telavancin against Gram-positive isolates from phase 3 studies of hospital-acquired pneumonia (ATTAIN) [abstract no. C1-149]. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2008 Oct 25–28; Washington, DC
Nichol KA, Vashisht R, Decorby MR, et al. Activity of telavancin against Gram-positive cocci isolated from Canadian hospitals: CANWARD 2007 [abstract no. C1-150]. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2008 Oct 25-28; Washington, DC
Saravolatz LD, Pawlak J, Johnson LB. Comparative activity of telavancin against isolates of community-associated methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother 2007 Aug; 60(2): 406–9
Fritsche TR, Jones RN. Antimicrobial activity of telavancin tested against contemporary Gram-positive pathogens: results from an international surveillance program (2007) [abstract no. C1-148]. 48th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2008 Oct 25–28; Washington, DC
Krause KM, Renelli M, Difuntorum S, et al. In vitro activity of telavancin against resistant Gram-positive bacteria. Antimicrob Agents Chemother 2008 Jul; 52(7): 2647–52
Rubino CM, Van Wart SA, Bhavnani SM, et al. Oritavancin population pharmacokinetics in healthy subjects and patients with complicated skin and skin structure infections or bacteremia. Antimicrob Agents Chemother 2009 Oct; 53(10): 4422–8
Anderson VR, Keating GM. Dalbavancin. Drugs 2008; 68(5): 639–48; discussion 49-51
Cavaleri M, Riva S, Valagussa A, et al. Pharmacokinetics and excretion of dalbavancin in the rat. J Antimicrob Chemother 2005 Mar; 55 Suppl. 2: ii31–5
Fetterly GJ, Ong CM, Bhavnani SM, et al. Pharmacokinetics of oritavancin in plasma and skin blister fluid following administration of a 200-milligram dose for 3 days or a single 800-milligram dose. Antimicrob Agents Chemother 2005 Jan; 49(1): 148–52
Lemaire S, Kosowska-Shick K, Julian K, et al. Activities of antistaphylococcal antibiotics towards the extracellular and intraphagocytic forms of Staphylococcus aureus isolates from a patient with persistent bacteraemia and endocarditis. Clin Microbiol Infect 2008 Aug; 14(8): 766–77
Gerber J, Smirnov A, Wellmer A, et al. Activity of LY333328 in experimental meningitis caused by a Streptococcus pneumoniae strain susceptible to penicillin. Antimicrob Agents Chemother 2001 Jul; 45(7): 2169–72
Shaw JP, Seroogy J, Kaniga K, et al. Pharmacokinetics, serum inhibitory and bactericidal activity, and safety of telavancin in healthy subjects. Antimicrob Agents Chemother 2005 Jan; 49(1): 195–201
Sun HK, Duchin K, Nightingale CH, et al. Tissue penetration of telavancin after intravenous administration in healthy subjects. Antimicrob Agents Chemother 2006 Feb; 50(2): 788–90
Lodise Jr TP, Gotfried M, Barriere S, et al. Telavancin penetration into human epithelial lining fluid determined by population pharmacokinetic modeling and Monte Carlo simulation. Antimicrob Agents Chemother 2008 Jul; 52(7): 2300–4
Stucki A, Gerber P, Acosta F, et al. Efficacy of telavancin against penicillin-resistant pneumococci and Staphylococcus aureus in a rabbit meningitis model and determination of kinetic parameters. Antimicrob Agents Chemother 2006 Feb; 50(2): 770–3
Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2009; 66(1): 82–98
McKay GA, Beaulieu S, Arhin FF, et al. Time-kill kinetics of oritavancin and comparator agents against Staphylococcus aureus, Enterococcus faecalis and Enterococcus faecium. J Antimicrob Chemother 2009; 63(6): 1191–9
Andes D, Craig WA. In vivo pharmacodynamic activity of the glycopeptide dalbavancin. Antimicrob Agents Chemother 2007 May; 51(5): 1633–42
Jabes D, Candiani G, Romano G, et al. Efficacy of dalbavancin against methicillin-resistant Staphylococcus aureus in the rat granuloma pouch infection model. Antimicrob Agents Chemother 2004 Apr; 48(4): 1118–23
Bowker KE, Noel AR, MacGowan AP. Pharmacodynamics of dalbavancin studied in an in vitro pharmacokinetic system. J Antimicrob Chemother 2006 Oct; 58(4): 802–5
Dunbar LM, Milata J, Fitzpatrick M, et al. Efficacy of oritavancin at single or infrequent doses for the treatment of complicated skin and skin structure infections [poster no. 1849]. 19th European Congress of Clinical Microbiology and Infectious Disease; 2009 May 16–19; Helsinki
Stryjewski ME, Graham DR, Wilson SE, et al. Telavancin versus vancomycin for the treatment of complicated skin and skin-structure infections caused by Gram-positive organisms. Clin Infect Dis 2008 Jun; 46(11): 1683–93
Greenblatt DJ, Zhao Y, Duan SX, et al. Effect of oritavancin on human cytochromes P450 in vitro [poster no. A1-1284]. 49th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2009 Sep 12-15; San Francisco (CA)
Acknowledgements
No sources of funding were used to assist in the preparation of this review. The authors have declared that they have no conflicts of interest that are directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Additional information
An Erratum for this chapter can be found at http://dx.doi.org/0012-6667/11/0005-0526/
An erratum to this article is available at http://dx.doi.org/10.2165/11591910-000000000-00000.
Rights and permissions
About this article
Cite this article
Zhanel, G.G., Calic, D., Schweizer, F. et al. New Lipoglycopeptides. Drugs 70, 859–886 (2010). https://doi.org/10.2165/11534440-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11534440-000000000-00000