Summary
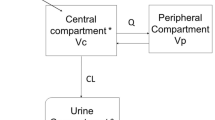
This open-label, multiple-dose study was intended to evaluate the pharmacokinetics of clarithromyin and azithromycin in plasma/serum, urine, and inflammatory and noninflammatory blister fluid, in 8 normal subjects. Co-modelling of the plasma/serum and urine data for each drug produced complex multicompartment models. Observed versus fitted drug concentration analyses evidenced a good fit of the data, despite moderate coefficient of variation values for all parameters. Blister fluid analyses demonstrated significant concentrating of both drugs in inflammatory versus noninflammatory conditions (areas under the blister fluid concentration versus time curves 31.6 versus 11.2 mg·h/L for clarithromycin and 2.88 versus 0.07 mg·h/L for azithromycin), although there was no significant difference in degree of accumulation between the agents. The results of this study are consistent with previous reports demonstrating white blood cell delivery of clarithromycin and azithromycin, and the concentrating of these agents in inflamed/infected areas.
Similar content being viewed by others
References
Peters DH, Clissold SP. Clarithromycin. A review of its antimicrobial activity, pharmacokinetic properties and therapeutics potential. Drugs 1992; 44: 117–64
Wollmer P, Pride NB, Rhodes CG, et al. Measurement of pulmonary erythromycin concentration in patients with lobar pneumonia by means of position tomography. Lancet 1982; 2: 1361–4
Kohna Y, Ohta K, Suwa T, et al. Autobacteriography studies of clarithromycin and erythromycin in mice. Antimicrob Agents Chemother 1990; 34: 562–7
Schentag JJ, Ballow CH. Tissue-directed pharmacokinetics. Am J Med 1991; 91 Suppl. 3A: 5S–11S
Baldwin DR, Wise R, Andrews JM, et al. Azithromycin concentrations at the sites of pulmonary infection. Eur Respir J 1990; 3: 886–90
Fish DN, Gotfried MH, Danziger LH, et al. Penetration of clarithromycin into lung tissues from patients undergoing lung resection. Antimicrob Agents Chemother 1994; 38: 876–8
Conte JE Jr, Golden JA, Duncan S, et al. Intrapulmonary pharmacokinetics of clarithromycin and of erythromycin. Antimicrob Agents Chemother 1995; 39: 334–8
Foulds G, Shepard RM, Johnson RB. The pharmacokinetics of azithromycin in human serum and tissues. J Antimicrob Chemother 1990; 25 Suppl A: 73–82
Ballow CH, Amsden GW, Forrest A, et al. Pharmacokinetics (Pks) of azithromycin in serum urine, inflammatory (IB) and non-inflammatory (NB) blisters [abstract]. Clin Pharmacol Ther 1993; 53: 211
Ballow CH, Amsden GW, Higher VS, et al. Concentration of azithromycin (A) in polymorphonuclear leukocytes (PMNs): role in tissue delivery [abstract 3274]. Presented at the 19th International Conference on Chemotherapy, Montreal, Quebec, Canada, 1995
D’Argenio DZ, Schumitzky A. ADAPTII users guide. Biomedical simulations resource. University of Southern California, Los Angeles, California, 1992
D’Argenio DZ, Schumitsky A. A program package for simulation and parameter estimation in pharmacokinetic systems. Comp Prog Biomed 1979; 9: 115–34
Yamaoka K, Terumichi N, Uno T. Application of Akaike’s Information Criterion (AIC) in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm 1978; 6 165–75
Amsden GW, Schentag JJ. Tables of antimicrobial agent pharmacology. In: Mandell GL, Bennet JE, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. New York: Churchill Livingstone, 1995: 492–528
Ballow CH, Amsden GW, Forrest A, et al. Comparison of the plasma, urine and blister fluid pharmacokinetics of clarithromycin (C) and azithromycin (A) in normal subjects. Presented at the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy, Orlando, Florida, 1994
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Amsden, G.W., Ballow, C.H. & Forrest, A. Comparison of the Plasma, Urine and Blister Fluid Pharmacokinetics of Clarithromycin and Azithromycin in Normal Subjects. Clin. Drug Invest. 13, 152–161 (1997). https://doi.org/10.2165/00044011-199713030-00005
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-199713030-00005