Abstract
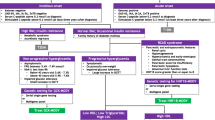
Maturity-onset diabetes of the young (MODY) is a dominantly inherited form of non-ketotic diabetes mellitus. It results from a primary defect of insulin secretion, and usually develops at childhood, adolescence, or young adulthood. MODY is a heterogeneous disease with regard to genetic, metabolic, and clinical features. All MODY genes have not been identified, but heterozygous mutations in six genes cause the majority of the MODY cases. By far MODY2 (due to mutations of the glucokinase gene) and MODY3 (due to mutations in hepatocyte nuclear factor-la) are the most frequent. As with MODY3, all the other MODY subtypes are associated with mutations in transcription factors. The clinical presentations of the different MODY subtypes differ, particularly in the severity and the course of the insulin secretion defect, the risk of microvascular complications of diabetes, and the defects associated with diabetes. Patients with MODY2 have mild, asymptomatic, and stable hyperglycemia that is present from birth. They rarely develop microvascular disease, and seldom require pharmacologic treatment of hyperglycemia. In patients with MODY3, severe hyperglycemia usually occurs after puberty, and may lead to the diagnosis of type 1 diabetes. Despite the progression of insulin defects, sensitivity to sulfonylureas may be retained in MODY3 patients. Diabetic retinopathy and nephropathy frequently occur in patients with MODY3, making frequent follow-up mandatory. By contrast, other risk factors are not present in patients with MODY and the frequency of cardiovascular disease is not increased. The clinical spectrum of MODY is wider than initially described, and might include multi-organ involvement in addition to diabetes. In patients with MODY5, due to mutations in hepatocyte nuclear factor-1β, diabetes is associated with pancreatic atrophy, renal morphologic and functional abnormalities, and genital tract and liver test abnormalities. Although MODY is dominantly inherited, penetrance or expression of the disease may vary and a family history of diabetes is not always present. Thus, the diagnosis of MODY should be raised in various clinical circumstances. Molecular diagnosis has important consequences in terms of prognosis, family screening, and therapy.

Similar content being viewed by others
References
Fajans SS. Scope and heterogeneous nature of MODY. Diabetes Care 1990; 13: 49–64
Froguel P. Nuclear factors and type 2 diabetes. Schweiz Med Wochenschr 1998; 128: 1936–9
Malecki MT, Jhala US, Antonellis A, et al. Mutations in NEUROD1 are associated with the development of type 2 diabetes mellitus. Nat Genet 1999; 23: 323–8
Fajans SS, Bell GI, Polonsky KS. Molecular mechanisms and clinical physiopathology of maturity-onset diabetes of the young. N Engl J Med 2001; 345: 971–80
Hattersley AT. Maturity-onset diabetes of the young: clinical heterogeneity explained by genetic heterogeneity. Diabet Med 1998; 15: 15–24
Frayling TM, Lingren CM, Chevre JC, et al. A genome-wide scan in families with maturity-onset diabetes of the young: evidence for further heterogeneity. Diabetes 2003; 52: 872–81
Gloyn AL. Glucokinase (GCK) mutations in hyper- and hypoglycemia: maturityonset diabetes of the young, permanent neonatal diabetes, and hyperinsulinemia of infancy. Hum Mutat 2003; 22: 353–62
Njolstad PR, Sovik O, Cuesta-Munoz A, et al. Neonatal diabetes due to complete glucokinase deficiency. N Engl J Med 2001; 344: 1588–92
Gloyn AL, Ellard S, Shield JP, et al. Complete glucokinase deficiency is not a common cause of permanent neonatal diabetes [letter]. Diabetologia 2002; 45: 290
Davis EA, Cuesta-Munoz A, Raoul M, et al. Mutants of glucokinase cause hypoglycaemia and hyperglycaemia syndromes and their analysis illuminates fundamental quantitative concepts of glucose homeostasis. Diabetologia 1999; 42: 1175–86
Gidh-Jain M, Takeda J, Xu LZ, et al. Glucokinase mutations associated with non-insulin dependent (type 2) diabetes mellitus have decreased enzymatic activity: implications for structure/function relationships. Proc Natl Acad Sci U S A 1993; 90: 1932–6
Velho G, Froguel P, Gloyn A, et al. Maturity-onset diabetes of the young type 2. In: Magnuson M, Matschinsky F, editors. Glucokinase and glycémic diseases: from basics to novel therapeutics. Basel: Karger, 2004: 42–64
Velho G, Froguel P, Clément K, et al. Primary pancreatic beta-cell secretory defect caused by mutations in the glucokinase in kindreds of maturity-onset diabetes of the young. Lancet 1992; 340: 444–8
Byrne MM, Sturis J, Clément K, et al. Insulin secretory abnormalities in subjects with hyperglycemia due to glucokinase mutations. J Clin Invest 1994; 93:1120–30
Pueyo ME, Clement K, Vaxillaire M, et al. Arginine-induced insulin release in glucokinase-deficient subjects. Diabetes Care 1994; 17: 1015–21
Sreenan SK, Cockburn BN, Baldwin AC, et al. Adaptation to hyperglycemia enhances insulin secretion in glucokinase mutant mice. Diabetes 1998; 47:1881–8
Velho G, Petersen KF, Perseghin G, et al. Impaired hepatic glycogen synthesis in glucokinase-deficient (MODY-2) subjects. J Clin Invest 1996; 98: 1755–61
Tappy L, Dussoix P, Iynedjian P, et al. Abnormal regulation of glucose output in maturity-onset diabetes of the young caused by a specific mutation of the glucokinase gene. Diabetes 1997; 46: 204–8
Clément K, Pueyo ME, Vaxillaire M, et al. Assessment of insulin sensitivity in glucokinase-deficient subjects. Diabetologia 1996; 39: 82–90
Froguel P, Zouali H, Vionnet N, et al. Familial hyperglycemia due to mutations in glucokinase: definition of a subtype of diabetes mellitus. N Engl J Med 1993; 328: 729–31
Hattersley AH, Beards F, Ballantyne E, et al. Mutations in the glucokinase gene of the fetus result in reduced birth weight. Nat Genet 1998; 19: 268–70
Velho G, Hattersley AT, Froguel P. Maternal diabetes alters birth weight in glucokinase-deficient (MODY2) kindred but has no influence on adult weight, height, insulin secretion or insulin sensitivity. Diabetologia 2000; 43: 1060–3
Terauchi Y, Kubota N, Tamemoto H, et al. Insulin effect during embryogenesis determines fetal growth: a possible molecular link between birth weight and susceptibility to type 2 diabetes. Diabetes 2000; 49: 82–6
Velho G, Blanché H, Vaxillaire M, et al. Identification of 14 new glucokinase mutations and description of the clinical profile of 42 MODY-2 families. Diabetologia 1997; 40: 217–24
Pearson ER, Velho G, Clark P, et al. β-cell genes and diabetes: quantitative and qualitative differences in the pathophysiology of hepatic nuclear factor-1α and glucokinase mutations. Diabetes 2001; 50Suppl. 1: S101–7
Ellard S, Beards F, Allen LI, et al. A high prevalence of glucokinase mutations in gestational diabetic subjects selected by clinical criteria. Diabetologia 2000; 43: 250–3
Vehlo G, Vaxillaire M, Boccio V, et al. Diabetes complications in NIDDM kindreds linked to the MODY3 locus on chromosome 12q. Diabetes Care 1996; 19: 915–9
Guenat E, Seematter G, Philippe J, et al. Counter-regulatory responses to hypoglycemia in patients with glucokinase gene mutations. Diabetes Metab 2000; 26: 377–84
Grimsby J, Sarabu R, Corbett WL, et al. Allosteric activators of glucokinase: potential role in diabetes therapy. Science 2003; 301: 370–3
Spyer G, Hattersley AT, Sykes JE, et al. Influence of maternal and fetal glucokinase mutations in gestational diabetes. Am J Obstet Gynecol 2001; 185: 240–1
Yamagata K, Oda N, Kaisaki PJ, et al. Mutations in the hepatocyte nuclear factor1α] gene in maturity-onset diabetes of the young (MODY3). Nature 1996; 384: 455–8
Velho G, Froguel P. Maturity-onset diabetes of the young (MODY), MOD Y genes, and non-insulin-dependent diabetes mellitus. Diabetes Metab 1997; 23: 34–7
Frayling TM, Bulman MP, Ellard S, et al. Mutations in the hepatocyte nuclear factor-1α gene are a common cause of maturity-onset diabetes of the young in the United Kingdom. Diabetes 1997; 46: 720–5
Cox RD, Souyham L, Hashim Y, et al. UKPDS 31: hepatocyte nuclear factor-1 alpha (the MODY3 gene) mutations in late onset type II diabetic patients in the United Kingdom. United Kingdom Prospective Diabetes Study. Diabetologia 1999; 42: 120–1
Mendel DB, Hansen LP, Graves MK, et al. HNF-1 alpha and HNF-1beta (vHNF-1) share dimerization and homeodomains, but not activation domains, and form heterodimers in vitro. Genes Dev 1991; 5: 1042–56
Kaisaki PJ, Menzel S, Lindner T, et al. Mutations in the hepatocyte nuclear factor-1α gene in MODY and early-onset NIDDM: evidence for a mutational hotspot in exon 4. Diabetes 1997; 46: 528–35
Gragnoli C, Lindner T, Cockburn BN, et al. Maturity-onset diabetes of the young due to a mutation in the hepatocyte nuclear factor-4 alpha binding site in the promoter of the hepatocyte nuclear factor-1 alpha gene. Diabetes 1997; 46: 1648–51
Godart F, Bellanné-Chantelot C, Clauin S, et al. Identification of seven novel nucleotide variants in the hepatocyte nuclear factor-1 α (TCF1) promoter region in MODY patients. Hum Mutat 2000; 15: 173–80
Pontoglio M, Barra J, Hadchouel M, et al. Hepatocyte nuclear factor-1 inactivation results in hepatic dysfunction, phenylketonuria, and renal Fanconi syndrome. Cell 1996; 84: 575–85
Shih DQ, Bussen M, Sehayek E, et al. Hepatocyte nuclear factor-1α is an essential regulator of bile acid and plasma cholesterol metabolism. Nat Genet 2001; 27: 375–82
Pontoglio M, Sreenan S, Roe M, et al. Defective insulin secretion in hepatocyte nuclear factor-1α-deficient mice. J Clin Invest 1998; 101: 2215–22
Dukes ID, Sreenan S, Roe MW, et al. Defective pancreatic β-cell glycolytic signaling in hepatocyte nuclear factor-1α-deficient mice. J Biol Chem 1998; 272: 24457–64
Wang H, Maechler P, Hagenfeldt KA, et al. Dominant-negative suppression of HNF-1 α function results in defective insulin gene transcription and impaired metabolism-secretion coupling in a pancreatic β-cell line. EMBO J 1998; 17: 6701–13
Okita K, Yang Q, Yamagata K, et al. Human insulin gene is a target of hepatocyte nuclear factor-1α (HNF-1α) and HNF-lβ. Biochem Biophys Res Commun 1999; 263: 566–9
Shih DQ, Screenan S, Munoz KN, et al. Loss of HNF-1α function in mice leads to abnormal expression of genes involved in pancreatic islet development and metabolism. Diabetes 2001; 50: 2472–80
Vaxillaire M, Pueyo ME, Clément K, et al. Insulin secretion and insulin sensitivity in diabetic and non-diabetic subjects with hepatic nuclear factor-1α (maturity-onset diabetes of the young-3) mutations. Eur J Endocrinol 1999; 141: 609–18
Yang Q, Yamagata K, Yamamoto K, et al. Structure/function studies of hepatocyte nuclear factor-1 alpha, a diabetes-associated transcription factor. Biochem Biophys Res Commun 1999; 266: 196–202
Yoshiuchi Y, Yamagata K, Yang Q, et al. Three new mutations in the hepatocyte nuclear factor-1 alpha gene in Japanese subjects with diabetes mellitus: clinical features and functional characterization. Diabetologia 1999; 42: 621–6
Harries LW, Hattersley AT, Ellard S. Messenger RNA transcripts of the hepatocyte nuclear factor-1α gene containing premature termination codons are subject to nonsense-mediated decay. Diabetes 2004; 53: 500–4
Ellard S. Hepatocyte nuclear factor-1 alpha (HNF-1 alpha) mutations in maturity-onset diabetes of the young. Hum Mutat 2000; 16: 377–85
Frayling TM, Evans JC, Bulman MP, et al. B-cell genes and diabetes: molecular and clinical characterization of mutations in transcription factors. Diabetes 2001; 50Suppl. 1: S94–100
Klupa T, Warram JH, Antonellis A, et al. Determinants of the development of diabetes (maturity-onset diabetes of the young-3) in carriers of HNF-1α mutations. Diabetes Care 2002; 25: 2292–301
Lehto M, Tuomi T, Mahtani MM, et al. Characterization of the MODY3 phenotype: early-onset diabetes caused by an insulin secretion defect. J Clin Invest 1997; 99: 582–91
Sumerly JF, Guenat E, Philippe J, et al. Glucose utilization and production in patients with maturity-onset diabetes of the young caused by a mutation of the hepatocyte nuclear factor-1α gene. Diabetes 1998; 47: 1459–63
Miedzybrodzka Z, Hattersley AT, Ellard S, et al. Non-penetrance in a MODY3 family with a mutation in the hepatocyte nuclear factor-1 alpha gene: implications for predictive testing. Eur J Hum Genet 1999; 7: 729–32
Stride A, Sepherd M, Frayling TM, et al. Intrauterine hyperglycemia is associated with an earlier diagnosis of diabetes in gene mutation carriers. Diabetes Care 2002; 25: 2287–91
Kim SH, Ma X, Klupa T, et al. Genetic modifiers of the age at diagnosis of diabetes (MODY3) in carriers of HNF-1α mutations map to chromosomes 5pl5, 9q22, and 14q24. Diabetes 2003; 52: 2182–6
Menzel R, Kaisaki PJ, Rjasanowski I, et al. A low renal threshold for glucose in diabetic patients with a mutation in the hepatocyte nuclear factor-1α (HNF-1α) gene. Diabet Med 1998; 15: 816–20
Pontoglio M, Prie D, Chenet C, et al. HNF-1α controls renal glucose reabsorption in mouse and man. EMBO Rep 2000; 11: 359–65
Owen KR, Stride A, Ellard S, et al. Etiological investigation in young adults presenting with apparent type 2 diabetes. Diabetes Care 2003; 26: 2088–93
Moller AM, Dalgaard LT, Pociot F, et al. Mutations in the hepatocyte nuclear factor-1α gene in Caucasian families originally classified as having type 1 diabetes. Diabetologia 1998; 41: 1528–31
Kawazaki E, Sera Y, Yamakawa K, et al. Identification and functional analysis of mutations in the hepatocyte nuclear factor-1 alpha gene in anti-islet autoantibody-negative Japanese patients with type 1 diabetes. J Clin Endocrinol Metab 2000; 85: 331–5
Owen KR, Sheperd M, Stride A, et al. Heterogeneity in young adult onset diabetes: aetiology alters clinical characteristics. Diabet Med 2002; 19: 758–61
Isomaa B, Henricsson M, Lehto M, et al. Chronic diabetic complications in patients with MODY3 diabetes. Diabetologia 1998; 41: 467–73
Pearson ER, Liddell WG, Shepherd M, et al. Sensitivity to sulphonylureas in patients with hepatocyte nuclear factor-1 alpha gene mutations: evidence for pharmacogenetics in diabetes. Diabet Med 2000; 17: 543–5
Pearson ER, Starkey BJ, Powell RJ, et al. Genetic cause of hyperglycaemia and response to treatment in diabetes. Lancet 2003; 362: 1275–81
Doria A, Yang Y, Malecki M, et al. Phenotypic characteristics of early-onset autosomal-dominant type 2 diabetes unlinked to known maturity-onset diabetes of the young (MODY) genes. Diabetes Care 1999; 22: 253–61
Shepherd M, Pearson ER, Houghton J, et al. No deterioration in glycemic control in HNF-1 alpha maturity-onset diabetes of the young following transfer from long-term insulin to sulphonylureas. Diabetes Care 2003; 26: 3191–2
Bluteau O, Jeannot E, Bioulac-Sage P, et al. Bi-allelic inactivation of TCF1 in hepatic adenomas. Nat Genet 2002; 32: 312–5
Bacq Y, Jacquemin E, Balabaud C, et al. Familial liver adenomatosis associated with hepatocyte nuclear factor-1 alpha inactivation. Gastroenterology 2003; 125: 1470–5
Reznik Y, Dao T, Coutant R, et al. Hepatocyte nuclear factor-1 alpha gene inactivation: co-segregation between liver adenomatosis and diabetes phenotypes in two maturity-onset diabetes of the young (MODY)3 families. J Clin Endocrinol Metab 2004; 89: 1476–80
Gragnoli C, Lindner T, Cockburn BN, et al. Maturity-onset diabetes of the young due to a mutation in the hepatocyte nuclear factor-4 alpha binding site in the promoter of the hepatocyte nuclear factor-1 alpha gene. Diabetes 1997; 46: 1648–51
Thomas H, Jaschkowitz K, Bulman M, et al. A distant upstream promoter of the HNF-4 alpha gene connects the transcription factors involved in maturity-onset diabetes of the young. Hum Mol Genet 2001; 10: 2089–97
Stoffel M, Duncan SA. The maturity-onset diabetes of the young (MODY1) transcription factor HNF-4 alpha regulates expression of genes required for glucose transport and metabolism. Proc Natl Acad Sci U S A 1997; 94: 13209–14
Parviz F, Matullo C, Garrison WD, et al. Hepatocyte nuclear factor-4α controls the development of a hepatic epithelium and liver morphogenesis. Nat Genet 2003; 34: 292–6
Stoffers DA, Ferrer J, Clarke WL, et al. Early-onset type II diabetes mellitus (MODY4) linked to IPF-1. Nat Genet 1997; 17: 138–9
Clocquet_AR, Egan JM, Stoffers DA, et al. Impaired insulin secretion and increased insulin sensitivity in familial maturity-onset diabetes of the young 4 (insulin promoter factor 1 gene). Diabetes 2000; 49: 1856–64
Cockburn BN, Bermano G, Boodram LL, et al. Insulin promoter factor-1 and diabetes in Trinidad: identification of a novel diabetes-associated mutation (E224K) in an Indo-Trinidadian family. J Clin Endocrinol Metab 2004; 89: 971–8
Shih DQ, Stoffel M. Dissecting the transcriptional network of pancreatic islets during development and differentiation. Proc Natl Acad Sci U S A 2001; 98: 14189–91
Reber M, Cereghini S. Variant hepatocyte nuclear factor 1 expression in the mouse genital tract. Mech Dev 2001; 100: 75–8
Coffinier C, Barra J, Babinet C, et al. Expression of the vHNF1/HNF1beta homeoprotein gene during mouse organogenesis. Mech Dev 1999; 89: 211–3
Coffinier C, Gresh L, Fiette L, et al. Bile system morphogenesis defects and liver dysfunction upon targeted deletion of HNF1beta. Development 2002; 129: 1829–38
Kolatsi-Joannou M, Bingham C, Ellard S, et al. Hepatocyte nuclear factor-1beta: a new kindred with renal cysts and diabetes and gene expression in normal human development. J Am Soc Nephrol 2001; 12: 2175–80
Gresh L, Fischer E, Reimann A, et al. A transcriptional network in polycystic kidney disease. EMBO J 2004; 23: 1657–68
Hiesberger T, Bai Y, Shao X, et al. Mutation of hepatocyte nuclear factor-1beta inhibits Pkhd1 gene expression and produces renal cysts in mice. J Clin Invest 2004; 113:814–25
Ward CJ, Hogan MC, Rossetti S, et al. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet 2002; 30: 259–69
Horikawa Y, Iwasaki N, Hara M, et al. Mutation in hepatocyte nuclear factor-1beta gene (TCF2) associated with MODY. Nat Genet 1997; 17: 384–5
Nishigori H, Yamada S, Kohama T, et al. Frameshift mutation, A263fsinsGG, in the hepatocyte nuclear factor-1beta gene associated with diabetes and renal dysfunction. Diabetes 1998; 47: 1354–5
Iwasaki N, Ogata M, Tomonaga H, et al. Liver and kidney function in Japanese patients with maturity-onset diabetes of the young. Diabetes Care 1998; 21: 2144–8
Lindner TH, Njolstad PR, Horikawa Y, et al. A novel syndrome of diabetes mellitus, renal dysfunction and genital malformation associated with a partial deletion of the pseudo-POU domain of hepatocyte nuclear factor-1beta. Hum Mol Genet 1999; 8: 2001–8
Bingham C, Ellard S, Allen L, et al. Abnormal nephron development associated with a frameshift mutation in the transcription factor hepatocyte nuclear factor-1beta. Kidney Int 2000; 57: 898–907
Bingham C, Bulman MP, Ellard S, et al. Mutations in the hepatocyte nuclear factor-1beta gene are associated with familial hypoplastic glomerulocystic kidney disease. Am J Hum Genet 2001; 68: 219–24
Iwasaki N, Okabe I, Momoi MY, et al. Splice site mutation in the hepatocyte nuclear factor-lbeta gene, IVS2nt + 1G > A, associated with maturity-onset diabetes of the young, renal dysplasia and bicornuate uterus. Diabetologia 2001; 44: 387–8
Carbone I, Cotellessa M, Barella C, et al. A novel hepatocyte nuclear factor-1beta (MODY-5) gene mutation in an Italian family with renal dysfunctions and early-onset diabetes. Diabetologia 2002; 45: 153–4
Yoshiuchi I, Yamagata K, Zhu Q, et al. Identification of a gain-of-function mutation in the HNF-1beta gene in a Japanese family with MODY. Diabetologia 2002; 45: 154–5
Bingham C, Ellard S, Cole TR, et al. Solitary functioning kidney and diverse genital tract malformations associated with hepatocyte nuclear factor-1beta mutations. Kidney Int 2002; 61: 1243–51
Montoli A, Colussi G, Massa O, et al. Renal cysts and diabetes syndrome linked to mutations of the hepatocyte nuclear factor-1beta gene: description of a new family with associated liver involvement. Am J Kidney Dis 2002; 40: 397–402
Furuta H, Furuta M, Sanke T, et al. Nonsense and missense mutations in the human hepatocyte nuclear factor-1beta gene (TCF2) and their relation to type 2 diabetes in Japanese. J Clin Endocrinol Metab 2002; 87: 3859–63
Waller SC, Rees L, Woolf AS, et al. Severe hyperglycemia after renal transplantation in a pediatric patient with a mutation of the hepatocyte nuclear factor-1beta gene. Am J Kidney Dis 2002; 40: 1325–30
Mache CJ, Preisegger KH, Kopp S, et al. De novo HNF-1beta gene mutation in familial hypoplastic glomerulocystic kidney disease. Pediatr Nephrol 2002; 17: 1021–6
Bellanné-Chantelot C, Chauveau D, Gautier JF, et al. Clinical spectrum associated with hepatocyte nuclear factor-1β mutations. Ann Intern Med 2004; 140: 510–7
Yamada S, Tomura H, Nishigori H, et al. Identification of mutations in the hepatocyte nuclear factor1lalpha gene in Japanese subjects with early-onset NIDDM and functional analysis of the mutant proteins. Diabetes 1999; 48: 645–8
Rizzoni G, Loirat C, Levy M, et al. Familial hypoplastic glomerulocystic kidney: a new entity? Clin Nephrol 1982; 18: 263–8
Bingham C, Ellard S, van’t Hoff WG, et al. Atypical familial juvenile hyperuricemic nephropathy associated with a hepatocyte nuclear factor-1beta gene mutation. Kidney Int 2003; 63: 1645–51
Acknowledgments
The authors have provided no information on sources of funding or on conflicts of interest directly relevant to the content of this review.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Timsit, J., Bellanné-Chantelot, C., Dubois-Laforgue, D. et al. Diagnosis and Management of Maturity-Onset Diabetes of the Young. Mol Diag Ther 4, 9–18 (2005). https://doi.org/10.2165/00024677-200504010-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00024677-200504010-00002