Abstract
Synopsis
Paclitaxel is a new anticancer agent with a novel mechanism of action. It promotes polymerisation of tubulin dimers to form microtubules and stabilises microtubules by preventing depolymerisation.
In noncomparative trials, continuous infusion of paclitaxel 110 to 300 mg/m2 over 3 to 96 hours every 3 to 4 weeks produced a complete or partial response in 16 to 48% of patients with ovarian cancer and 25 to 61.5% of patients with metastatic breast cancer, many of whom were refractory to treatment with cisplatin or doxorubicin, respectively. 23 to 100% of patients with ovarian cancer achieved complete or partial responses with paclitaxel in combination with cisplatin, carboplatin, cyclophosphamide, altretamine and/or doxorubicin. Similarly, response rates of 30 to 100% were observed with paclitaxel plus doxorubicin, cisplatin, mitoxantrone and/or cyclophosphamide in patients with metastatic breast cancer. Comparative trials in patients with advanced ovarian cancer showed paclitaxel therapy to produce greater response rates than treatment with parenteral hydroxyurea (71 vs 0%) or cyclophosphamide (when both agents were combined with cisplatin) [79 vs 63%]. Paclitaxel was also more effective than mitomycin in 50 patients with previously untreated breast cancer (partial response in 20 vs 4% of patients).
Paclitaxel therapy also produced promising results in patients with advanced squamous cell carcinoma of the head and neck, malignant melanoma, advanced non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), germ cell cancer, urothelial cancer, oesophageal cancer, non-Hodgkin’s lymphoma or multiple myeloma, and was successfully combined with cisplatin, carboplatin and/or etoposide in patients with NSCLC, SCLC or advanced squamous cell carcinoma of the head and neck.
Hypersensitivity reactions were initially a concern with administration of paclitaxel, although current dosage regimens have reduced the incidence of these events to less than 5%. The major dose-limiting adverse effects of paclitaxel are leucopenia (neutropenia) and peripheral neuropathy. Other haematological toxicity was generally mild. Cardiac toxicity was reported in small numbers of patients and most patients developed total alopecia.
Several aspects of paclitaxel use remain to be clarified, including the optimal treatment schedule and infusion time, confirmation of the tolerability profile and efficacy of combination regimens in an expanded range of malignancies. Long term follow-up of paclitaxel recipients will also allow the effects of the drug on patient survival to be determined. Nevertheless, paclitaxel is a promising addition to the current therapies available, with significant activity reported in patients with advanced ovarian or breast cancer or other types of tumour. The drug has activity as salvage or initial therapy, alone or in combination with currently used agents, against a number of cancers. In particular, paclitaxel should be considered a first-line agent in women with ovarian cancer refractory to cisplatin and as a second-line agent for the treatment of metastatic breast cancer if doxorubicin is ineffective.
Pharmacodynamic Properties
Paclitaxel, a new anticancer agent first isolated from the bark of Taxus brevifolia, has a wide spectrum of antineoplastic activity. It has in vitro cytotoxicity against human ovary, breast, cervical, pancreas, prostate, head and neck, colon, gastric, bladder, lung and CNS cancers, melanoma, hepatoma and leukaemia cell lines, often at concentrations lower than those achieved in the serum of patients. Similarly, fresh human lung, ovarian, breast and endometrial cancer cells, sarcoma and leukaemia cells are sensitive to paclitaxel. Increasing exposure time to paclitaxel appears to increase the activity of the drug. However, resistant tumour cell lines and fresh tumour isolates have been reported.
In general, activity of paclitaxel in vitro was greater than that of tiazofurine, cisplatin, etoposide, doxorubicin or fluorouracil against human tumours and similar or less than (generally 2 to 4 times lower) that of docetaxel, as measured by mean concentrations producing 50% inhibition of cell growth. In vitro, synergistic interactions generally occurred when exposure to paclitaxel was initiated up to 48 hours prior to cisplatin, when cells were exposed to doxorubicin prior to incubation with higher concentrations of paclitaxel, when cells pretreated with edatrexate were exposed to paclitaxel, when 24-hour exposure to paclitaxel preceded incubation with melphalan, thiotepa or fluorouracil or when paclitaxel was combined with tiazofurine, calcitriol, vinorelbine or estramustine. However, the order in which drugs were added appears to be an important determinant of synergism or antagonism. Paclitaxel also enhances the effects of irradiation against many cell lines in vitro and a mammary carcinoma in mice. It has also been evaluated in combination with irradiation in patients with non-small cell lung cancer (NSCLC) or brain tumours.
Three or 4 weeks’ treatment with intraperitoneal or subcutaneous paclitaxel 12.5 mg/kg for 4 or 5 consecutive days out of seven induced total tumour regression in nude mice with human glioblastoma (9 to 20%), breast cancer (80%), lung cancer (14%) or tongue cancer xenografts (17%). No regressions were observed in mice with a fast-growing gliosarcoma or endometrial or ovarian cancer xenografts. In other studies, intravenously or intraperitoneally administered paclitaxel was active against intraperitoneal human ovarian and prostate cancer xenografts transplanted into mice, but was inactive in 3 of 4 subcutaneous human pancreatic adenocarcinoma models.
The mechanisms of inherent or acquired resistance to paclitaxel have not yet been fully elucidated, but increased expression of the multidrug resistant gene (mdr-1) and alterations in α- or β-tubulin have been implicated. Clinically relevant concentrations of polyoxyethylated castor oil (polyoxyl 35 castor oil), the base used clinically in formulations of paclitaxel, reverses resistance of some cells to the drug, as does co-incubation with verapamil, quinidine or cyclosporin. In mice, definite cross-resistance developed in amsacrine-resistant leukaemia cell lines, marginal cross-resistance developed in doxorubicin-, dactinomycin- or mitoxantrone-resistant cell lines, but no cross-resistance was noted in camptothecin-, melphalan-, cisplatin-, cytarabine- or methotrexate-resistant cell lines. Similarly, no cross-resistance to cisplatin was noted in ovarian or lung cancer cell lines, although cross-resistance to doxorubicin and etoposide did occur in the lung cancer cell lines.
Reversible neurotoxicity develops in some patients treated with paclitaxel. Neurological examination shows the most common abnormality to be reduced or absent ankle reflexes. When paclitaxel is injected directly into the sciatic nerve of rats, microtubules proliferate in both axons and Schwann cells at the expense of most other organelles and result in congestion and dilation of Schwann cell bodies and development of significant stretches of naked axons, some of which display unusual axon-Schwann cell relationships. Remyelination appears dependent on decreasing axon diameter. Apparent normalisation of Schwann cells, axons and endoneural cells generally occurs 6 months after injection of paclitaxel in animals.
Paclitaxel also produces concentration-dependent suppression of human peripheral blood mononuclear and natural killer cell cytotoxicity against cancer cell lines. These effects are reduced by pretreatment with interleukin-2. In contrast, stimulated, but not unstimulated, release of the proinflammatory cytokines interleukin-1β and tumour necrosis factor-α from human mononuclear phagocytes is dose-dependently enhanced by paclitaxel. Microtubule-associated functions of neutrophils are also inhibited by the drug.
Pharmacokinetic Properties
Plasma concentrations of paclitaxel increase throughout 6- or 24-hour infusions and begin to decline immediately upon cessation of the infusion. Although maximum plasma concentration (Cmax) and area under the concentration-time curve (AUC) were dose-related in small numbers of patients who received paclitaxel 120 to 300 mg/m2 as a 3-/6- or 24-hour continuous infusion, the pharmacokinetic behaviour of paclitaxel appears to be nonlinear. Administration of paclitaxel 135 to 350 mg/m2 produces mean steady-state plasma drug concentrations higher [0.20 to 8.54 mg/L (0.23 to 10 μmol/L)] than concentrations producing antimicrotubule effects in vitro (at least 0.1 μmol/L). Paclitaxel is cleared rapidly from plasma initially, eliminated over a prolonged period (4.3 to 49.76 hours), and is extensively protein bound (88 to 98%). The apparent volume of distribution is large and is correlated with administered drug dose. Paclitaxel does not appear to easily cross the blood-brain barrier in humans. In rodents, paclitaxel is predominantly distributed to the liver, lung, spleen, adrenal and salivary glands, heart, muscle, kidneys, stomach, intestine and pancreas but not the nervous system or testes.
Peak intraperitoneal concentrations of 16 to 277 mg/L (19 to 324 μmol/L) occur 30 to 60 minutes after completion of administration of intraperitoneal paclitaxel 25 to 175 mg/m2. Clearance from the peritoneal cavity is slow (mean clearance of 0.42 L/m2/day and half-life of 73.4 hours), and peritoneal exposure to paclitaxel appears to be 336 to 2890 times greater than systemic exposure.
Mean total body clearance ranges from 8.04 to 23.55 L/h/m2 following a 3- to 24-hour infusion of paclitaxel 15 to 275 mg/m2 and is not correlated with dose. Hepatic metabolism and biliary clearance appear to be the major means of elimination of paclitaxel. Five metabolites of paclitaxel have been identified in human bile, of which 2 are monohydroxylated and 1 is a dihydroxylated derivative. The major metabolite of paclitaxel isolated in human liver microsomes is 6μ-hydroxytaxol. Urinary clearance of paclitaxel was minimal (16% or less). Clearance of paclitaxel was reduced in 9 patients with liver tumours and aspartate aminotransferase levels at least 1.5 times normal, compared with values in 13 patients without hepatic involvement (20.16 vs 28.26 L/h/m2). Dialysis did not appear to significantly alter pharmacokinetic parameters of paclitaxel in 1 patient. The pharmacokinetic profile of paclitaxel in children does not appear to differ from that observed in adults.
Mean clearance rates of paclitaxel are reduced by prior cisplatin administration compared with the reverse schedule (19.26 vs 24.3 L/h/m2). Concomitant administration of doxorubicin and paclitaxel did not alter steady-state concentration or clearance of either drug compared with monotherapy. Fluconazole and ketoconazole inhibited, and cimetidine or erythromycin hade little or no effect, on the metabolism of paclitaxel in vitro.
The severity and incidence of adverse effects appeared to be correlated with AUC, steady-state concentration, the duration plasma paclitaxel concentrations remained above a certain level and absolute dose of paclitaxel in some, but not all, studies.
Therapeutic Potential
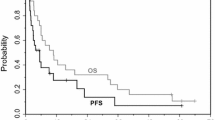
In phase II studies, paclitaxel was usually administered as a 24-hour continuous infusion to patients with a good or excellent performance status. However, 3-hour infusions have been successfully used in patients with advanced ovarian or breast cancer. Noncomparative trials showed continuous intravenous infusion of paclitaxel 110 to 300 mg/m2 over 3, 24 or 96 hours every 3 to 4 weeks to produce a complete or partial response in 16 to 48% of patients with ovarian cancer and 20 to 61.5% of patients with metastatic breast cancer. The median time to disease progression was longer in patients with ovarian or breast cancer treated with paclitaxel 175 versus 135 mg/m2 and in those with ovarian cancer treated with a 3- versus 24-hour infusion. Median survival was 9 months in 652 evaluable patients with ovarian cancer treated with 24-hour infusions of paclitaxel 135 mg/m2 every 3 weeks. In general, patients with ovarian cancer responded to treatment with paclitaxel 135 to 300 mg/m2 after a median of 9.2 weeks and duration of response ranged from 2 to 18.6 (median 7.2) months. Intraperitoneal paclitaxel produced a limited response in a small number of patients with major manifestations of residual ovarian or breast cancer confined to the peritoneal cavity enrolled in a phase I trial.
Many patients with ovarian or breast cancer who responded to therapy with paclitaxel had been refractory to cisplatin or doxorubicin therapy, respectively. Response was not correlated with estrogen receptor status in patients with breast cancer.
Phase I studies conducted in small numbers of patients showed paclitaxel in combination with cisplatin and cyclophosphamide to produce objective responses in 58% of patients with newly diagnosed advanced ovarian cancer and with cisplatin to produce complete or partial responses in all 5 patients with suboptimally debulked ovarian cancer. Objective responses were also achieved by patients with previously treated ovarian cancer who received paclitaxel and cyclophosphamide (47%), paclitaxel and oral altretamine (23%) or paclitaxel and doxorubicin 25 mg/m2 (40%); however, only small numbers of patients were enrolled in these studies. Paclitaxel produced greater clinical response rates than treatment with parenteral hydroxyurea (71 vs 0%), and when combined with cisplatin, it was more effective than cyclophosphamide plus cisplatin (79 vs 63%) in patients with ovarian cancer. In the latter study, median duration of progression-free survival was 17.9 months for paclitaxel recipients and 13.8 months for women treated with cyclophosphamide.
In small numbers of patients with breast cancer, combination therapy with paclitaxel and doxorubicin produced response rates of 44.5 to 100%. Paclitaxel plus cyclophosphamide produced a partial response in 30 or 62% of patients with metastatic disease and the combination of paclitaxel and cisplatin produced partial responses in 52 or 94% of patients.
Paclitaxel, alone or in combination with cisplatin, produced complete or partial responses in 21 or 37% and 33% of patients, respectively, with advanced or metastatic squamous cell carcinoma of the head and neck. Treatment with paclitaxel also resulted in objective responses in patients with oesophageal cancer (32%), non-Hodgkin’s lymphoma (42%) or previously untreated multiple myeloma (23%). Promising results were obtained with 24-hour infusions of paclitaxel 200 to 275 mg/m2 in patients with malignant melanoma (response rates of 12 to 33%), cisplatin-resistant germ cell cancer (response rate of 24%), previously untreated advanced transitional cell carcinoma of the urothelium (response rate of 42%), advanced previously untreated NSCLC [response in 21 or 24% patients, median survival of 24.1 or 40 weeks and 1-year survival rate of 42%] or previously untreated small cell lung cancer (partial responses in 34 or 40.5%). However, paclitaxel was less effective in patients with previously treated NSCLC. When combined with cisplatin or carboplatin, paclitaxel produced complete or partial responses in 25 to 50% and 22 to 80%, respectively, of small numbers of patients with NSCLC. Combination therapy with paclitaxel, cisplatin and etoposide was very promising in 7 patients with previously untreated NSCLC; 6 patients had a partial response after 2 courses of therapy. Similarly, all 8 patients with SCLC achieved a complete or partial response after treatment with paclitaxel, carboplatin and etoposide.
Tolerability
Severe acute hypersensitivity reactions occur in some patients treated with paclitaxel, in many cases necessitating therapeutic intervention and/or discontinuation of therapy. However, the incidence of these reactions has been reduced to less than 5% subsequent to the introduction of premedication with dexamethasone and histamine H1- and H2-receptor antagonists. Hypersensitivity reactions frequently include dyspnoea, hypotension, manifestations of angioedema, urticaria, flushing and/or erythematous rash and usually occur during administration of the first or second dose of paclitaxel, in most instances within 10 minutes of initiating the drug infusion.
The dose-limiting toxicity of paclitaxel is generally neutropenia or leucopenia, although administration of granulocyte colony-stimulating factor (G-CSF) and a reduction in infusion time from 24 to 3 hours reduces the incidence of this toxicity. In clinical trials, grade 4 leucopenia or granulocytopenia developed in 16 to 100% of patients treated with paclitaxel 135 to 250 mg/m2 over 24 hours. As the duration of neutropenia is usually only 3 to 10 days and is not cumulative, myelosuppression is not dose limiting in many patients. However, 21 to 62% of patients who received the drug as a 24-hour infusion were hospitalised because of febrile neutropenia, while sepsis during neutropenia resulted in death in about 1.3% of patients. Neutropenia tended to worsen when cisplatin therapy preceded a 24-hour infusion of paclitaxel, or when a 24-hour infusion of paclitaxel was administered prior to doxorubicin or cyclophosphamide, when compared with the reverse schedules. Although grade 3 or 4 thrombocytopenia and anaemia occurs infrequently with paclitaxel 135 to 350 mg/m2 (0 to 24% of patients), and in some studies thrombocytopenia did not occur, many patients develop mild manifestations of this toxicity.
Dose-related peripheral neuropathy can also be dose-limiting, with grade 3 or 4 toxicity affecting 2 to 27% of patients treated with paclitaxel. Neuropathy follows a ‘stocking and glove’ distribution, mainly involves neurosensory manifestations and tends to be symmetrical. However, in isolated instances patients have developed motor abnormalities. Peripheral neurotoxicity tended to occur more frequently in patients with pre-existing neuropathy or other risk factors for neuropathy in a small number of trials. Equivocal results were obtained with respect to the cumulative nature of paclitaxel-induced neuropathy. Myalgia or arthralgia also develop 2 to 6 days after administration of paclitaxel in 14 to 100% of patients. Although these symptoms resolve over 2 to 7 days, they can be extremely distressing and dose limiting in some patients. Optic nerve damage has also been reported in 9 of 47 patients receiving a 3-hour infusion of paclitaxel 175 or 225 mg/m2 in 1 trial.
Paclitaxel causes transient asymptomatic bradycardia in some patients, but serious dysrhythmias rarely develop. However, significant cardiovascular events occurred in small numbers of patients treated with paclitaxel in clinical trials. These included myocardial infarction, atrial fibrillation, mild congestive heart failure, ventricular and supraventricular tachycardia, ventricular arrhythmia, Wenckebach syndrome, sudden death, hypotension, asymptomatic T-wave inversion, atrioventricular block and asymptomatic sinus arrest.
Other adverse effects associated with paclitaxel include total alopecia (in almost all patients), grade 3 or 4 mucositis (1.4 to 30% of patients), predominantly mild nausea and vomiting and local venous toxicity (4 to 64%). Many additional usually mild effects (taste impairment, diarrhoea, anorexia, sore throat, stomatitis, fatigue, fever, oedema, hypotension, hypomagnesaemia, dyspnoea, headache, facial flushing, phlebitis, transient azotaemia and transient hepatocellular dysfunction) have also been reported in patients receiving the drug. In addition, grand mal seizures, unexplained reversible renal insufficiency, typhlitis (when paclitaxel was combined with doxorubicin) or cutaneous radiation recall reaction have been reported rarely. Age, prior therapy or the total cumulative dose of paclitaxel received appeared to have little effect on the tolerability profile of the drug.
Dosage and Administration
As paclitaxel is poorly soluble in water, it is formulated in a vehicle of 50% polyoxyethylated castor oil and 50% alcohol (ethanol).
Although adequate dose-response trials have not been completed for paclitaxel, a dosage of 135 to 175 mg/m2, administered as a 3- or 24-hour intravenous infusion every 3 weeks, appears to be effective in patients with metastatic ovarian cancer. A 3-hour infusion of paclitaxel 175 mg/m2 appears effective in patients with metastatic breast cancer after failure of previous antineoplastic chemotherapy. Subsequent courses of paclitaxel should be administered only when neutrophil and platelet counts are adequate, and dosage should be reduced by 20% if patients experience severe neutropenia or severe peripheral neuropathy.
In clinical trials, higher doses of paclitaxel could be administered with G-CSF without an increase in the incidence of haematological toxicity, but the incidence of peripheral neuropathy increased. If a 24-hour infusion of paclitaxel is to be administered with cisplatin, paclitaxel should be administered first. Although recommendations are not available concerning the use of paclitaxel in elderly patients or children, clinical studies have included patients aged from 2 to 88 years, many of whom were elderly, and used no special dosages in these patients. However, children tolerated higher dosages of paclitaxel than adults in dose-ranging studies.
Recommendations state that all patients should receive premedication with oral dexamethasone (or clemastine), and intravenous diphenhydramine and cimetidine or ranitidine before paclitaxel to reduce the risk of hypersensitivity reactions. Continuous cardiac monitoring is not necessary during treatment with paclitaxel, except in patients with serious pre-existing conduction abnormalities. Although few data are available concerning the use of paclitaxel in patients with liver impairment, lower dosages of the drug should be administered to these patients.
Similar content being viewed by others
References
Kohler DR, Goldspiel BR. Paclitaxel (taxol). Pharmacotherapy 1994; 14: 3–34
Manfredi JJ, Horwitz SB. Taxol: an antimitotic agent with a new mechanism of action. Pharmacol Ther 1984; 25: 83–125
Rao S, Band Horwitz S, Ringel I. Direct photoaffinity labeling of tubulin with taxol. J Natl Cancer Inst 1992; 84: 785–8
Rao S, Krauss NE, Heerding JM, et al. 3′-(p-Azidobenzamido)taxol photolabels the N-terminal 31 amino acids of β-tubulin. J Biol Chem 1994; 269: 3132–4
Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature 1979; 277: 665–7
Schiff PB, Horwitz SB. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci U S A 1980; 77: 1561–5
Schiff PB, Horwitz SB. Taxol assembles tubulin in the absence of exogenous guanosine 5′-triphosphate or microtubule-associated proteins. Biochemistry 1981; 20: 3247–52
Kumar N. Taxol-induced polymerization of purified tubulin. J Biol Chem 1981; 256: 10435–41
Dye RB, Fink SP, Williams Jr RC. Taxol-induced flexibility of microtubules and its reversal by MAP-2 and Tau. J Biol Chem 1993; 268: 6847–50
Roberts JR, Rowinsky EK, Donehower RC, et al. Demonstration of the cell cycle positions of taxol-induced ‘asters’ and ‘bundles’ by sequential measurements of tubulin immunoflu-orescence, DNA content, and autoradiographic labeling of taxol-sensitive and -resistant cells. J Histochem Cytochem 1989; 37: 1659–65
Stearns ME, Wang M. Taxol blocks processes essential for prostate tumor cell (PC-3 ML) invasion and metastases. Cancer Res 1992; 52: 3776–81
Rowinsky EK, Donehower RC, Jones RJ, et al. Microtubule changes and cytotoxicity in leukemic cell lines treated with taxol. Cancer Res 1988; 48: 4093–100
Diaz JF, Andreu JM. Assembly of purified GDP-tubulin into microtubules induced by taxol and taxotere: reversibility, ligand stoichiometry, and competition. Biochemistry 1993; 32: 2747–55
Manfredi JJ, Parness J, Horwitz SB. Taxol binds to cellular microtubules. J Cell Biol 1982; 94: 688–96
Stracke ML, Soroush M, Liotta LA, et al. Cytoskeletal agents inhibit motility and adherence of human tumor cells. Kidney Int 1993; 43: 151–7
Cook JA, Fisher J, Teague D, et al. Sequence dependence of paclitaxel combined with cisplatin or alkylators in human cancer cells [abstract 1975]. Proc Am Assoc Cancer Res 1994; 35: 332
Chi C-W, Chang Y-F, Li L, et al. Taxol induced cytotoxicity in a human hepatoma cell line Hep3B [abstract 2759]. Proc Am Assoc Cancer Res 1994; 35: 462
Liebmann J, Cook JA, Lipschultz C, et al. The influence of Cremophor EL on the cell cycle effects of paclitaxel (Taxol®) in human tumor cell lines. Cancer Chemother Pharmacol 1994; 33: 331–9
Choy H, Rodriguez FF, Koester S, et al. Investigation of taxol as a potential radiation sensitizer. Cancer 1993; 71: 3774–8
Long BH, Fairchild CR. Paclitaxel inhibits progression of mitotic cells to G1 phase by interference with spindle formation without affecting other microtubule functions during anaphase and telephase. Cancer Res 1994; 54: 4355–61
Jordan MA, Toso RJ, Thrower D, et al. Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc Natl Acad Sci USA 1993; 90: 9552–6
Bhalla K, Ibrado AM, Tourkina E, et al. Taxol induces inter-nucleosomal DNA fragmentation associated with programmed cell death in human myeloid leukemia cells. Leukemia 1993; 7: 563–8
Bleiler JK, Tang C, Lutzky J, et al. Taxol induced apoptosis in human myeloma cells [abstract 2221]. Blood 1993; 82: 559A
Li X, Gong J, Feldman E, et al. Apoptotic cell death during treatment of leukemias. Leuk Lymphoma 1994; 13 Suppl. 1: 65–70
Liu Y, Hill CE, Bhalla K, et al. Evidence for involvement of tyrosine phosphorylation in apoptosis induced by taxol in a human ovarian tumor cell line [abstract 1863]. Proc Am Assoc CancerRes 1994; 35: 313
Saunders DE, Lawrence WD, Christensen C, et al. Taxol-induced apoptosis in MCF-7 breast cancer cells [abstract 1888]. Proc Am Assoc Cancer Res 1994; 35: 317
Ibrado AM, Ponnathpur V, Reed J, et al. Protein kinase C and tyrosine kinase activities affect taxol induced apoptosis in human leukemic cells [abstract 1866]. Proc Am Assoc Cancer Res 1994; 35: 314
Koechli OR, Sevin B-U, Perras JP, et al. Characteristics of the combination paclitaxel plus doxorubicin in breast cancer cell lines analyzed with the ATP-cell viability assay. Breast Cancer Res Treat 1993; 28: 21–7
Untch M, Sevin B-U, Perras JP, et al. Evaluation of paclitaxel (taxol), cisplatin, and the combination paclitaxel-cisplatin in ovarian cancer in vitro with the ATP cell viability assay. Gynecol Oncol 1994; 53: 44–9
Untch M, Untch A, Seven B-U, et al. Comparison of paclitaxel and docetaxel (Taxotere) in gynecologic and breast cancer cell lines with the ATP-cell viability assay. Anticancer Drugs 1994; 5: 24–30
Silvestrini R, Zaffaroni N, Orlandi L, Oriana S. In vitro cytotoxic activity of Taxol® and taxotere on primary cultures and established cell lines of human ovarian cancer. Stem Cells 1993; 11: 528–35
Vanhoefer U, Harstrick A, Wilke H, et al. Taxol in combination with cisplatin, etoposide and 5-fluourouracil in gastric cancer cell lines [abstract 508]. Ann Hematol 1993; 67 Suppl.: A129
Taniki T, Prajda N, Hata Y, et al. Synergistic action of taxol and tiazofurin in human ovarian, pancreatic and lung carcinoma cells [abstract 1769]. In: Proceedings of the 84th Annual Meeting of the American Association for Cancer Research; 1993 May 19-22; Orlando. 1993: 297
Kelland LR, Abel G. Comparative in vitro cytotoxicity of taxol and Taxotere against cisplatin-sensitive and -resistant human ovarian carcinoma cell lines. Cancer Chemother Pharmacol 1992; 30: 444–50
Kiyozuka Y, Nishimura H, Murakami F, et al. Paradoxical result in evaluating cytotoxicity of taxol in MTT assay on ovarian cancer cells. Nippon Gan Chiryo Gakkai Shi 1993; 28: 1824–35
Braakhuis BJM, Hill BT, Dietel M, et al. In vitro antiproliferative activity of docetaxel (Taxotere®), paclitaxel (Taxol®) and cisplatin against human tumour and normal bone marrow cells. Anticancer Res 1994; 14: 205–8
Lopes NM, Adams EG, Pitts TW, et al. Cell kill kinetics and cell cycle effects of taxol on human and hamster ovarian cell lines. Cancer Chemother Pharmacol 1993; 32: 235–42
Liebmann JE, Cook JA, Lipschultz C, et al. Cytotoxic studies of paclitaxel (Taxol®) in human tumour cell lines. Br J Cancer 1993; 68: 1104–9
Cook JC, Teague D, Mitchell JB. Differential effects of taxol and vinblastine in rodent and human cell lines [abstract 1725]. In: Proceedings of the 84th Annual Meeting of the American Association for Cancer Research; 1993 May 19-22; Orlando. 1993:290
Schultz RM, Merriman RL, Toth JE, et al. Evaluation of new anticancer agents against the MIA PaCa-2 and PANC-1 human pancreatic carcinoma xenografts. Oncol Res 1993; 5: 223–8
Speicher LA, Barone L, Tew KD. Combined antimicrobial activity of estramustine and taxol in human prostatic carcinoma cell lines. Cancer Res 1992; 52: 4433–40
Röyttä M, Laine K-M, Härkönen P. Morphological studies on the effect of taxol on cultured human prostatic cancer cells. Prostate 1987; 11: 95–106
Figg WD, Thibault A, McCall NA, et al. The in vitro activity of taxol on three hormone refractory prostate cancer (HRPC) cell lines, PC3, DU145, and PC3M [abstract 2570]. Proc Am Assoc Cancer Res 1994; 35: 431
Casazza AM, Rose WC, Fairchild CR, et al. Preclinical biological profile of taxol (T) and taxotere (TXT) [abstract 2267]. In: Proceedings of the 84th Annual Meeting of the American Association for Cancer Research; 1993 May 19–22; Orlando. 1993: 380
Niell HB, Rangel C, Miller A, et al. The activity of antimicrotubular agents in human bladder tumor cell lines (HBTCL) [abstract 1207]. In: Proceedings of the 84th Annual Meeting of the American Association for Cancer Research; 1993 May 19–22; Orlando. 1993: 202
Rangel C, Niell HB. The activity of taxol in human bladder tumor cell lines (HBTCL) [abstract 2915]. Proc Am Assoc Cancer Res 1992; 33: 488
Ohnoshi T, Takigawa N, Ueoka H, et al. Antitumour activity of taxol against human lung cancer cell lines. Haigan 1993; 33: 343–8
Huber MH, Murphy WK, Hong WK, et al. Characterization of taxol cytotoxicity against human non-small cell lung cancer (NSCLC) cell lines [abstract 2079]. In: Proceedings of the 84th Annual Meeting of the American Association for Cancer Research; 1993 May 19–22; Orlando. 1993: 349
Helson L, Helson C, Malik S, et al. Taxol effects on human neuroblastoma, primitive neuroectodermal tumor, melanoma, & glioblastoma cell lines [abstract 370]. Proc Am Soc Clin Oncol 1993; 12: 143
Leonard CE, Prasad K, Kumar R, et al. In vitro effect of taxol on squamous cell carcinoma of the head and neck [abstract 2424]. Proc Am Assoc Cancer Res 1994; 35: 407
Tishler RB, Geard CR, Hall EJ, et al. Taxol sensitizes human astrocytoma cells to radiation. Cancer Res 1992; 52: 3495–7
Helson L. Cephalomannine and 10-deacetyltaxol cytotoxicity in human glial and neuroblastoma cell lines. Int J Oncol 1993; 2: 297–9
Cahan M, Walter KA, Colvin OM, Brem H. Cytotoxicity of taxol in vitro against human and rat malignant brain tumors. Cancer Chemother Pharmacol 1994; 33: 441–4
Alberts DS, Garcia D, Fanta P, et al. Comparative cytotoxicities of taxol & taxotere in vitro against fresh human ovarian cancers [abstract 719]. Proc Am Soc Clin Oncol 1992; 11: 226
Zoli W, Savini S, Bajorko P, et al. In vitro evaluation of taxotere and taxol on primary cell cultures of human breast cancer [abstract]. In Proceedings of the Pisa Symposia in Oncology-Breast Cancer: from Biology to Therapy; Oct 19–21; Pisa. 1992: 68
Maguire YP, Lieu KL, Bartels P. In vitro test for fresh human tumors to taxol [abstract 581]. In: Proceedings of the 84th Annual Meeting of the American Association for Cancer Research; 1993 May 19–22; Orlando, 1993: 98
Rowinsky EK, Burke PJ, Karp JE, et al. Phase I and pharmacodynamic study of taxol in refractory acute leukemias. Cancer Res 1989; 49: 4640–7
Vogel M, Hilsenbeck SG, Depenbrock H, et al. Preclinical activity of taxotere (RP 56976, NSC 628503) against freshly explanted clonogenic human tumour cells: comparison with taxol and conventional antineoplastic agents. Eur J Cancer A 1993; 29A: 2009–14
Massad LS, Williams S, Mutch DG. Cell lines derived from endometrial cancers are resistant to lysis by taxol. Gynecol Oncol 1993; 49: 131–2
Matsuoka H, Furusawa M, Tomoda H, et al. Difference in cytotoxicity of paclitaxel against neoplastic and normal cells. Anticancer Res 1994; 14: 163–8
Georgiadis MS, Russell E, Johnson BE. Prolonging the exposure of human lung cancer cell lines to paclitaxel improves the cytotoxicity [abstract 2028]. Proc Am Assoc Cancer Res 1994; 35: 341
Hanauske A-R, Degen D, Hilsenbeck SG, et al. Effects of Taxotere and taxol on in vitro colony formation of freshly explanted human tumor cells. Anticancer Drugs 1992; 3: 121–4
Jekunen AP, Christen RD, Shalinsky DR, et al. Synergistic interaction between cisplatin and taxol in human ovarian carcinoma cells in vitro. Br J Cancer 1994; 69: 299–306
Lee KB, Parker RJ, Dabholkar M, et al. Taxol effect on cisplatin sensitivity and cisplatin cellular accumulation in human ovarian cancer cells [abstract 2144]. In: Proceedings of the 84th Annual Meeting of the American Association for Cancer Research; 1993 May 19–22; Orlando. 1993: 355
Rowinsky EK, Citardi MJ, Noe DA, et al. Sequence-dependent cy totoxic effects due to combinations of cisplatin and the antimicrotubule agents taxol and vincristine. J Cancer Res Clin Oncol 1993; 119: 727–33
Kern DH, Morgan CR. Apparent in vitro antagonism between cisplatin and taxol [abstract 1788]. In: Proceedings of the 84th Annual Meeting of the American Association for Cancer Research; 1993 May 19–22; Orlando. 1993: 300
Gercel-Taylor C, Taylor D, Owens K. Effect of sequencing with taxol on the cisplatin sensitivity of ovarian cancer cell lines [abstract 1959]. Proc Am Assoc Cancer Res 1994; 35: 329
Hahn SM, Liebmann JE, Cook J, et al. Taxol in combination with doxorubicin or etoposide. Possible antagonism in vitro. Cancer 1993; 72: 2705–11
Waud.WR, Schmid SM, Plowman J. In vitro and in vivo combination chemotherapy evaluations of taxol with doxorubicin or topotecan [abstract]. Poster from the Second National Cancer Institute Workshop on Taxol and Taxus; 1992 Sep 23–24; Alexandria USA. 1992
Saunders DE, Christensen C, LoRusso PM, et al. Inhibition of ovarian carcinoma cells by taxol combined with vitamin D and adriamycin [abstract 2641]. Proc Am Assoc Cancer Res 1992; 33: 442
Hahn S, Liebmann J, Fisher J, et al. Taxol in combination with doxorubicin (DOX), etoposide (VP-16), and m-AMSA: possible antagonism in vitro [abstract]. Poster from the Second National Cancer Institute Workshop on Taxol and Taxus; 1992 Sep 23–24; Alexandria USA. 1992
Geoffroy F, Patel M, Ren Q-F, et al. Interaction of fluorouracil and paclitaxel in MCF-7 human breast carcinoma cells [abstract 1962]. Proc Am Assoc Cancer Res 1994; 35: 330
Adams DJ. Synergy of navelbine-taxol combination treatment in two human breast cancer cell lines [abstract 327]. Proc Am Assoc Cancer Res 1994; 35: 327
Chou T-C, Otter GM, Sirotnak FM. Combined effects of edatrexate with taxol or taxotere against breast cancer cell growth [abstract 1783]. In: Proceedings of the 84th Annual Meeting of the American Association for Cancer Research; 1993 May 19–22; Orlando. 1993: 300
Hata Y, Sandler AB, Loehrer PJ, et al. Gallium nitrate and paclitaxel are synergistic in breast and additive in ovarian carcinoma cells [abstract 1980]. Proc Am Assoc Cancer Res 1994; 35: 333
Rose WC. Taxol: a review of its preclinical in vivo antitumor activity. Anticancer Drugs 1992; 3: 311–21
Riondel J, Jacrot M, Fessi H, et al. Effects of free and liposome-encapsulated taxol on two brain tumors xenografted into nude mice. In Vivo 1992; 6: 23–8
Riondel J, Jacrot M, Picot F, et al. Therapeutic response to taxol of six human tumors xenografted into nude mice. Cancer Chemother Pharmacol 1986; 17: 137–42
Walter KA, Cahan MA, Gur A, et al. Interstitial taxol delivered from a biodegradable polymer implant against experimental malignant glioma. Cancer Res 1994; 54: 2207–11
Sharma A, Mayhew E, Straubinger RM. Antitumor effect of taxol-containing liposomes in a taxol-resistant murine tumor model. Cancer Res 1993; 53: 5877–81
Nicoletti MI, Lucchini V, Massazza G, et al. Antitumour activity of taxol (NSC-125973) in human ovarian carcinomas growing in the peritoneal cavity of nude mice. Ann Oncol 1993; 4: 151–5
Sternberg CN, Sordillo PP, Cheng E, et al. Evaluation of new anticancer agents against human pancreatic carcinomas in nude mice. Am J Clin Oncol 1987; 10: 219–21
LoRusso PM, Demchik LL, Plowman J, et al. Preclinical activity and toxicity of taxol combinations [abstract 1794]. In: Proceedings of the 84th Annual Meeting of the American Association for Cancer Research; 1993 May 19–22; Orlando. 1993: 301
Ozols RE. Chemotherapy for advanced epithelial ovarian cancer. Hematol Oncol Clin North Am 1992; 6: 879–94
Barber HRK. New frontiers in ovarian cancer diagnosis and management. Yale J Biol Med 1991; 64: 127–41
Pazdur R, Kudelka AP, Kavanagh JJ, et al. The taxoids: paclitaxel (Taxol®) and docetaxel (Taxotere®). Cancer Treat Rev 1993; 19: 351–86
Uziely B, Delaflor-Weiss E, Lenz HJ, et al. Paclitaxel (taxol) in refractory breast cancer: response correlates with low levels of MDR1 gene expression [abstract 104]. Proc Am Soc Clin Oncol 1994; 13:75
Bhalla K, Huang Y, Tang C, et al. Isolation and characterization of taxol resistant human myeloid leukemia cells [abstract 1820]. Proc Am Assoc Cancer Res 1993; 34: 306
Roy SN, Horwitz SB. A phosphoglycoprotein associated with taxol resistance in J774.2 cells. Cancer Res 1985; 45: 3856–63
Ozols RE. Advances in the chemotherapy of gynecologic malignancies. Hematol Oncol 1992; 10: 43–51
Williams S, Rader JS, Herzog TJ, et al. Multiple mechanisms are involved in resistance of ovarian carcinoma cells to taxol. Gynecol Oncol 1993; 49: 110
Eck L, Pavich D, Fruehauf JP. MDR-1 expression by human ovarian tumors is asssoicated with taxol resistance [abstract 1388]. Proc Am Assoc Cancer Res 1993; 34: 232
Gupta RS. Taxol resistant mutants of Chinese hamster ovary cells: genetic biochemical, and cross-resistance studies. J Cell Physiol 1983; 114: 137–44
Cabrai FR, Brady RC, Schibier MJ. A mechanism of cellular resistance to drugs that interfere with microtubule assembly. Ann N Y Acad Sci 1986; 466: 745–56
Ohta S, Nishio K, Ohmori T, et al. A taxol resistant human small cell lung cancer cell line is partially dependent on taxol for its growth [abstract 1810]. Proc Am Assoc Cancer Res 1993; 34: 304
Roberts JR, Allison DC, Donehower RC, et al. Development of polyploidization in taxol-resistant human leukemia cells in vitro. Cancer Res 1990; 50: 710–6
Liebmann JE, Hahn SM, Cook JA, et al. Glutathione depletion by L-buthionine sulfoximine antagonizes taxol cytotoxicity. Cancer Res 1993; 53: 2066–70
Waud WR, Gilbert KS, Harrison Jr SD, et al. Cross-resistance of drug-resistant murine P388 leukemias to taxol in vivo. Cancer Chemother Pharmacol 1992; 31: 255–7
Chervinsky DS, Brecher ML, Hoelcle MJ. Cremophor-EL enhances taxol efficacy in a multi-drug resistant Cl300 neuroblastoma cell line. Anticancer Res 1993; 13: 93–6
Webster L, Linsenmeyer M, Millward M, et al. Measurement of cremphor EL following taxol: plasma levels sufficient to reverse drug exclusion mediated by the multidrug-resistant phenotype. J Natl Cancer Inst 1993; 85: 1685–90
Tolcher AW, Cowan KH, Solomon D, et al. A phase I study of paclitaxel (T) with R-verapamil (RV) in metastatic breast cancer (MBC) [abstract 349]. Proc Am Soc Clin Oncol 1994; 13: 139
Lehnert M, Emerson S, Dalton WS, et al. Reversal of P-glycoprotein-associated resistance to taxol and taxotere [abstract]. Abstract from the Second National Cancer Institute Workshop on Taxol and Taxus; 1992 Sep 23–24; Alexandria, 1992
Yang JM, Sommer S, Hait WN. Reversal of taxol resistance in vitro and in vivo by trans-flupenthixol and cyclosporin A [abstract 2116]. Proc Am Assoc Cancer Res 1994; 35: 355
Sinclair WK. Cyclic X-rays responses in mammalian cells in vitro. Radiat Res 1968; 33: 620–43
Glantz L, Choy H, Stops E, et al. Histological response analysis of brain tumors following concurrent taxol and radiation therapy [abstract 522]. Proc Am Soc Clin Oncol 1994; 13: 183
Glantz M, Walhberg L, Kim L, et al. Phase I study of weekly taxol and concurrent cranial radiation in adults with primary brain tumors [abstract 521]. Proc Am Soc Clin Oncol 1994; 13: 183
Hei TK, Hall EJ. Taxol, radiation, and oncogenic transformation. Cancer Res 1993; 53: 1368–72
Liebmann J, Cook JA, Fisher J, et al. In vitro studies of taxol as a radiation sensitizer in human tumor cells. J Natl Cancer Inst 1994; 86: 441–6
Lokesnwar BL, Ferrell SM, Block NL. Taxol enhances radiation-induced toxicity in prostatic tumor cells and delays tumor growth [abstract 2148]. Proc Am Assoc Cancer Res 1993; 34: 360
Tishler RB, Schiff PB, Geard CR, et al. Taxol: a novel radiation sensitizer. Int J Radiat Oncol Biol Phys 1992; 22: 613–7
Chang A, Keng P, Sobel S, et al. Interaction of radiation (XRT) and taxol [abstract 2168]. Proc Am Assoc Cancer Res 1993; 34: 364
Steren A, Sevin B-U, Perras J, et al. Taxol sensitizes human ovarian cancer cells to radiation. Gynecol Oncol 1993; 48: 252–8
Steren A, Sevin B-U, Perras J, et al. Taxol as a radiation sensitizer: a flow cytometric study. Gynecol Oncol 1993; 50: 89–93
Milas L, Hunter NR, Mason KA, et al. Enhancement of tumor radioresponse of a murine mammary carcinoma by paclitaxel. Cancer Res 1994; 54: 3506–10
Kirsch J, Zutra A, Littauer UZ. Characterization and intracellular distribution of microtubule-associated protein 2 in differentiating human neuroblastoma cells. J Neurochem 1990; 55: 1031–41
Chaudhry V, Rowinsky EK, Cornblath DR, et al. Taxol-induced neurotoxicity: sensorimotor neuropathy and myopathy [abstract P225]. Ann Neurol 1992; 32: 286
Postma TJ, Liefting AJM, Heimans JJ, et al. Neurotoxicity in patients with breast cancer (BC) and ovarian cancer (OC) treated with taxol [abstract 56]. Proc Am Soc Clin Oncol 1993; 12: 64
Rowinsky EK, Chaudhry V, Forastiere AA, et al. Phase I and pharmacologic study of paclitaxel and cisplatin with granulocyte colony-stimulating factor: neuromuscular toxicity is dose-limiting. J Clin Oncol 1993; 11: 2010–20
Lipton RB, Apfel SC, Dutcher JP, et al. Taxol produces a predominantly sensory neuropathy. Neurology 1989; 39: 368–73
New P, Barohn R, Gales T, et al. Taxol neuropathy after long-term administration [abstract 1226]. Proc Am Assoc Cancer Res 1991; 32: 205
Apfel SC, Lipton RB, Arezzo JC, et al. Nerve growth factor prevents toxic neuropathy in mice. Ann Neurol 1991; 29: 87–90
Hamers FPT, Pette C, Neijt JP, et al. The ACTH-(4-9) analog, ORG 2766, prevents taxol-induced neuropathy in rats. Eur J Pharmacol 1993; 233: 177–8
Peterson ER, Crain SM. Nerve growth factor attenuates neurotoxic effects of taxol on spinal cord-ganglion expiants from fetal mice. Science 1982; 217: 377–9
Röyttä M, Horwitz SB, Raine CS. Taxol-induced neuropathy: short-term effects of local injection. J Neurocytol 1984; 13: 685–701
Röyttä M, Raine CS. Taxol-induced neuropathy: further ultrastructural studies of nerve fibre changes in situ. J Neurocytol 1985; 14: 157–75
Röyttä M, Raine CS. Taxol-induced neuropathy: chronic effects of local injection. J Neurocytol 1986; 15: 483–96
Komiya Y. Changes in fast axonal transport by taxol injected subepineurally into the rat sciatic nerve. Neurosci Res 1992; 14: 159–65
Vuorinen V, Röyttä M, Raine CS. The long-term cellular response to taxol in peripheral nerve: Schwann cell and endoneurial cell changes. J Neurocytol 1989; 18: 785–94
Chuang LT, Lotzová E, Cook KR, et al. Effect of new investigational drug Taxol on oncolytic activity and stimulation of human lymphocytes. Gynecol Oncol 1993; 49: 291–8
Allen JN, Moore SA, Wewers MD. Taxol enhances but does not induce interleukin-lβ and tumor necrosis factor-α production. J Lab Clin Med 1993; 122: 374–81
Ding AH, Porteu F, Sanchez E, et al. Shared actions of endotoxin and taxol on TNF receptors and TNF release. Science 1990; 248: 370–2
Bogdan C, Ding A. Taxol, a micro tubule-stabilizing antineoplastic agent, induces expression of tumor necrosis factor a and interleukin-1 in macrophages. J Leukoc Biol 1992; 52: 119–21
Manthey CL, Brandes ME, Perera PY, et al. Taxol increases steady-state levels of lipopolysaccharide-inducible genes and protein-tyrosine phosphorylation in murine macrophages. J Immunol 1992; 149: 2459–65
Bottex-Gauthier C, Condemine F, Picot F, et al. Effects of taxol on the macrophage function. Interactions with some immunological parameters. Immunopharmacol Immunotoxicol 1992; 14: 39–61
Manthey CL, Quershi N, Stütz PL, et al. Lipopolysaccharide antagonists block taxol-induced signaling in murine macrophages. J Exp Med 1993; 178: 695–702
Williams S, Mutch DG, Xu L, et al. Divergent effects of taxol on tumor necrosis factor-α-mediated cytolysis of ovarian carcinoma cells. Am J Obstet Gynecol 1992; 167: 1870–6
Robert RL, Nath J, Friedman MM, et al. Effects of taxol on human neutrophils. J Immunol 1982; 129: 2134–41
Benis R, Mattson P. Microtubules, organelle transport, and steroidogenesis in cultured adrenocortical tumor cells. I. An ultrastructural analysis of cells in which basal and ACTH-induced steroidogenesis was inhibited by taxol. Tissue Cell 1989; 21: 479–94
Benis R, Mattson P. Microtubules, organelle transport, and steroidogenesis in cultured adrenocortical tumor cells. II. Reversibility of taxol’s inhibition of basal and ACTH-induced steroidogenesis is unaccompanied by reversibility of taxolinduced changes in cell ultrastructure. Tissue Cell 1989; 21: 687–98
Rainey WE, Kramer RE, Mason JI, et al. The effects of taxol, a microtubule-stabilizing drug, on steroidogenic cells. J Cell Physiol 1985; 123: 17–24
Huizing MT, Keung ACF, Rosing H, et al. Pharmacokinetics of paclitaxel and metabolites in a randomized comparative study in platinum-pretreated ovarian cancer patients. J Clin Oncol 1993; 11: 2127–35
Kearns C, Gianni L, Vigano L, et al. Non-linear pharmacokinetics of taxol in humans [abstract 341]. Proc Am Soc Clin Oncol 1993; 12: 135
Tamura T, Sasaki Y, Nishiwaki Y, et al. Phase I and pharmacokinetic study of paclitaxel administered by 3-hour infusion [abstract 322]. Proc Am Soc Clin Oncol 1994; 13: 132
Tamura T, Sasaki Y, Shinkai T, et al. Phase I and pharmacokinetic study of taxol by a 24-hour intravenous infusion [abstract 371]. Proc Am Soc Clin Oncol 1993; 12: 143
Wiernik PH, Schwartz EL, Strauman JJ, et al. Phase I clinical and pharmacokinetic study of taxol. Cancer Res 1987; 47: 2486–93
Schiller JH, Storer B, Tutsch K, et al. Phase I trial of 3-hour infusion of paclitaxel with or without granulocyte colony-stimulating factor in patients with advanced cancer. J Clin Oncol 1994; 12: 241–8
Wiernik PH, Schwartz EL, Einzig A, et al. Phase I trial of taxol given as a 24-hour infusion every 21 days: responses observed in metastatic melanoma. J Clin Oncol 1987; 5: 1232–9
Jamis-Dow CA, Klecker RW, Sarosy G, et al. Steady-state plasma concentrations and effects of taxol for a 250 mg/m2 dose in combination with granulocyte-colony stimulating factor in patients with ovarian cancer. Cancer Chemother Pharmacol 1993; 33: 48–52
Brown T, Havlin K, Weiss G, et al. A phase I trial of taxol given by a 6-hour intravenous infusion. J Clin Oncol 1991; 9: 1261–7
Longnecker SM, Donehower RC, Cates AE, et al. High-performance liquid Chromatographic assay for taxol in human plasma and urine and pharmacokinetics in a phase I trial. Cancer Treat Rep 1987; 71: 53–9
Keung ACF, Kaul S, Pinedo HM, et al. Pharmacokinetics of Taxol® given by 3-h or 24-h infusion to patients with ovarian carcinoma [abstract 321]. Proc Am Soc Clin Oncol 1993; 12: 130
Kumar GN, Walle UK, Bhalla KN, et al. Binding of taxol to human plasma, albumin and α1-acid glycoprotein. Res Commun Chem Pathol Pharmacol 1993; 80: 337–44
Markman M, Rowinsky E, Hakes T, et al. Phase I trial of intraperitoneal taxol: a Gynecologic Oncology Group study. J Clin Oncol 1992; 10: 1485–91
Francis P, Rowinsky E, Hakes T, et al. Phase I trial of weekly intraperitpneal (IP) taxol in patients (pts) with residual ovarian carcinoma (OC): a GOG study [abstract 813]. Proc Am Soc Clin Oncol 1993; 12: 257
Helson L, Puccio M, Ostrow S, et al. Phase I taxol: fractionated Q4D brief-infusional schedule in advanced solid tumors [abstract 448]. Proc Am Soc Clin Oncol 1994; 13: 163
Klecker RW, Jamis-Dow CA, Egorin MJ, et al. Distribution and metabolism of 3H-taxol in the rat [abstract 2268]. Proc Am Assoc Cancer Res 1993; 34: 380
Lesser GJ, Grossman SA, Eller S, et al. Distribution of 3H-taxol in the nervous system (NS) and organs of rats [abstract 441]. Proc Am Soc Clin Oncol 1993; 12: 160
Gaver RC, Cheng T, Puhl RJ, et al. Tissue distribution of 14C-paclitaxel in the rat [abstract 2541]. Proc Am Assoc Cancer Res 1994; 35: 426
Fujita H, Okamoto M, Takao A, et al. Pharmacokinetics of taxol (BMS-181339) in experimental animals [abstract]. Abstract from the 18th International Congress of Chemotherapy; 1993 Jun 27–Jul 2; Stockholm, 1993.
Kearns C, Gianni L, Capri G, et al. A comprehensive pharmacokinetic (PK) model of paclitaxel and 6-OH paclitaxel with application to pharmacodynamic (PD) relationships in humans [abstract 332]. Proc Am Soc Clin Oncol 1994; 13: 134
Venook AP, Egorin M, Brown TD, et al. Paclitaxel (taxol) in patients with liver dysfunction (CALGB 9264) [abstract 350]. Proc Am Soc Clin Oncol 1994; 13: 139
Grem JL, Tutsch KD, Simon KJ, et al. Phase I study of taxol administered as a short iv infusion daily for 5 days. Cancer Treat Rep 1987; 71: 1179–84
Walle T, Bhalla KN, Walle UK, et al. Taxol disposition in humans after tritium-labeled drug [abstract 404]. Proc Am Soc Clin Oncol 1994; 13: 152
Wright M, Monsarrat B, Alvinerie P, et al. Hepatic metabolism and biliary excretion of taxol [abstract]. Abstract from the Second National Cancer Institute Workshop on Taxol and Taxus; 1992 Sep 23–24: Alexandria, 1992
Kumar GN, Oatis JEJ, Thornburg KR, et al. 6α-Hydroxytaxol: isolation and identification of the major metabolite of taxol in human liver microsomes. Drug Metab Dispos 1994; 22: 177–9
Cresteil T, Monsarrat B, Alvinerie P, et al. Taxol metabolism by human liver microsomes: identification of cytochrome P450 isozymes involved in its biotransformation. Cancer Res 1994; 54: 386–92
Kumar GN, Walle UK, Walle T. Cytochrome P450 3A-mediated human liver microsomal taxol 6α-hydroxylation. J Pharmacol Exp Ther 1994; 268: 1160–5
Sonnichsen DS, Hurwitz CA, Pratt CB, et al. Saturable pharmacokinetics and paclitaxel pharmacodynamics in children with solid tumors. J Clin Oncol 1994; 12: 532–8
Seibel N, Ames M, Ivy P, et al. Phase I and pharmacologic study of paclitaxel in refractory pediatric leukemia: a Childrens Cancer Group study [abstract 1068]. Proc Am Soc Clin Oncol 1993; 13: 324
Wilson WH, Berg SL, Bryant G, et al. Paclitaxel in doxorubicin-refractory or mitoxantrone-refractory breast cancer: a phase I/II trial 96-hour infusion. J Clin Oncol 1994; 12: 1621–9
Rowinsky EK, Gilbert MR, McGuire WP, et al. Sequences of taxol and cisplatin: a phase I and pharmacologie study. J Clin Oncol 1991; 9: 1692–703
Berg SL, Cowan KH, Balis FM, et al. Pharmacokinetics of taxol and doxorubicin administered alone and in combination by continuous 72-hour infusion. J Natl Cancer Inst 1994; 86: 143–5
Reed E, Sarosy G, Jamis-Dow C, et al. Cimetidine does not influence taxol steady-state plasma levels [abstract 2353]. Proc Am Assoc Cancer Res 1993; 34: 395
Slichenmyer W, McGuire W, Donehower R, et al. Pharmacologic and toxic effects of various histamine-2 (H2A) antagonists in taxol premedication regimens [abstract 440]. Proc Am Soc Clin Oncol 1993; 12: 160
Jamis-Dow CA, Klecker RW, Katki AG, et al. Metabolism of taxol by human liver microsomes and effect of inhibitors [abstract 2198]. Proc Am Assoc Cancer Res 1993; 34: 369
Seidman AD, Barrett S, Tong W, et al. Pharmacokinetic (PK) & pharmcodynamic (PD) evaluation of 3 hour taxol infusion in patients (pts) with metastatic breast cancer (MBC) [abstract 403]. Proc Am Soc Clin Oncol 1994; 13: 152
Einzig AI, Wiernik PH, Sasloff J, et al. Phase II study and long-term follow-up of patients treated with taxol for advanced ovarian adenocarcinoma. J Clin Oncol 1992; 10: 1748–53
Reichman BS, Seidman AD, Crown JPA, et al. Paclitaxel and recombinant human granulocyte colony-stimulating factor as initial chemotherapy for metastatic breast cancer. J Clin Oncol 1993; 11: 1943–51
Ettinger DS, Finkelstein DM, Sarma R, et al. Phase II study of taxol in patients (pts) with extensive-stage small cell lung cancer (SCLC): an Eastern Cooperative Oncology Group Study [abstract 1094]. Proc Am Soc Clin Oncol 1993; 12: 329
Kohn EC, Sarosy G, Bicher A, et al. Dose-intense taxol: high response rate in patients with platinum-resistant recurrent ovarian cancer. J Natl Cancer Inst 1994; 86: 18–24
Horikoshi N, Ogawa M, Inoue K, et al. Pharmacokinetics of a 24 hour infusion of taxol [abstract 382]. Proc Am Soc Clin Oncol 1993; 12: 146
Donehower RC, Rowinsky EK, Grochow LB, et al. Phase I trial of taxol in patients with advanced cancer. Cancer Treat Rep 1987; 71: 1171–7
Spriggs DR, Tondini C. Taxol administered as a 120 hour infusion. Invest New Drugs 1992; 10: 275–8
Kris MG, O’Connell JP, Gralla RJ, et al. Phase I trial of taxol given as a 3-hour infusion every 21 days. Cancer Treat Rep 1986; 70: 605–7
Ohnuma T, Zimet AS, Coffey VA, et al. Phase I study of taxol in a 24-hour infusion schedule [abstract 662]. Proc Am Assoc Cancer Res 1985; 26: 167
Hainsworth JD, Hopkins L, Thomas M, et al. Taxol administered by one-hour infusion: preliminary results of a phase I/II study comparing two dose schedules [abstract 413]. Proc Am Soc Clin Oncol 1994; 13: 155
Legha SS, Tenney DM, Krakoff IR. Phase I study of taxol using a 5-day intermittent schedule. J Clin Oncol 1986; 4: 762–6
Stemmer S, Jones R, Bearman S, et al. Intensive taxol/cyclophosphamide/cisplatin (taxol/CPA/cDDP) with autologous hematopoietic cell support (AHCS) [abstract 222]. Proc Am Soc Clin Oncol 1994; 13: 105
Shea T, Graham M, Steagall A, et al. Multiple cycles of high dose taxol plus carboplatin with G-CSF (filgrastim) and peripheral blood progenitor cell (PBPC) support [abstract 395]. Proc Am Soc Clin Oncol 1994; 13: 150
Creaven PJ, Pendyala L, Wilkes JD, et al. Taxol (T) and carboplatin (C) ± GCSF in advanced solid tumors: a phase I and pharmacokinetic study [abstract 461]. Proc Am Soc Clin Oncol 1994; 13: 167
Peres EA, Coe T, Turrell C, et al. Phase I trial of oral etoposide plus intravenous taxol in advanced solid tumors [abstract 383]. Proc Am Soc Clin Oncol 1994; 13: 147
Lilenbaum RC, Rosner GL, Ratain MJ, et al. Phase I study of taxol and topotecan in patients with advanced solid tumors (CALBG 9362) [abstract 319]. Proc Am Soc Clin Oncol 1994; 13: 131
Hudes G, Obasaju C, McAleer C, et al. Phase I pharmacologie study of 96-hour infusional taxol combined with estramustine [abstract 465]. Proc Am Soc Clin Oncol 1994; 13: 168
Huber MH, Lee JS, Newman RA, et al. Phase I study of methotrexate (M) and paclitaxel (P) in patients (pts) with solid tumors [abstract 1217]. Proc Am Soc Clin Oncol 1994; 13: 361
Diamandidis DT, Lee JS, Shin DM, et al. Phase I study of taxol and edatrexate (EDAM) chemotherapy in solid tumors [abstract 453]. Proc Am Soc Clin Oncol 1994; 13: 165
Fennelly DM, Rigas JR, Chou D, et al. Phase I trial of edatrexate plus paclitaxel using an administration schedule with demonstrated in vitro synergy [abstract 1232]. Proc Am Soc Clin Oncol 1994; 13: 365
Bhalla KN, Kumar GN, Walle T, et al. Phase I and pharmacokinetic trial of a 3 hour infusion of taxol (T), cisplatin (CP) plus 5-fluorouracil (FU) in advanced solid tumors [abstract 454]. Proc Am Soc Clin Oncol 1994; 13: 165
Choy H, Akerley W, Safarin H, et al. A phase I trial of concurrent weekly taxol administered as a 3-hour infusion and radiation therapy for advanced non-small cell lung cancer [abstract 1210]. Proc Am Soc Clin Oncol 1994; 13: 360
Holmes FA, Walters RS, Theriault RL, et al. Phase II trial of taxol, an active drug in the treatment of metastatic breast cancer. J Natl Cancer Inst 1991; 83: 1797–805
Eisenhauer EA, ten Bokkel Huinink WW, Swenerton KD, et al. European-Canadian randomized trial of taxol in relapsed ovarian cancer: high vs low dose and long vs short infusion. J Clin Oncol. In press
Rowinsky EK, Donehower RC. Taxol: twenty years later, the story unfolds. J Natl Cancer Inst 1991; 83: 1778–81
Thigpen JT, Blessing JA, Vance RB, et al. Chemotherapy in ovarian carcinoma: present role and future prospects. Semin Oncol 1989; 16 Suppl. 6: 58–65
Ozols RF. Ovarian cancer, part II: treatment. Curr Probl Cancer 1992; 16: 61–126
Markman M. Taxol: an important new drug in the management of epithelial ovarian cancer. Yale J Biol Med 1991; 64: 583–90
Allen DG, Baak J, Belpomme D, et al. Advanced epithelial ovarian cancer: 1993 consensus statements. Ann Oncol 1993; 4 Suppl. 4: S83–S88
Athanassiou A, Pectasides D, Varthalitis I, et al. Taxol (T) patients (pts) with cis(C)/carbo(CA)platin-refractory ovarian carcinoma (OC) [abstract 870]. Proc Am Soc Clin Oncol 1994; 13: 271
McGuire WP, Rowinsky EK, Rosenshein NB, et al. Taxol: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Ann Intern Med 1989; 111: 273–9
Sarosy G, Kohn E, Stone DA, et al. Phase I study of taxol and granulocyte colony-stimulating factor in patients with refractory ovarian cancer. J Clin Oncol 1992; 10: 1165–70
Thigpen T, Blessing J, Ball H, et al. Phase II trial of taxol as second-line therapy for ovarian carcinoma: a Gynecologic Oncology Group study [abstract 604]. Proc Am Soc Clin Oncol 1990; 9: 156
Trimble EL, Adams JD, Vena D, et al. Paclitaxel for platinumrefractory ovarian cancer: results from the first 1,000 patients registered to national Cancer Institute Treatment Referral Center 9103. J Clin Oncol 1993; 11: 2405–10
Tropé C, Bertelsen K, Simonsen E, et al. A phase II, multicenter, non-randomised study of paclitaxel (taxol) in patients with previously untreated FIGO stage III suboptimally resected ovarian cancer [abstract]. Bristol-Myers Squibb (New Jersey) data on file. 1994
Salmi T, Bertelsen K, Björkholm E, et al. A phase II, multicenter, non-randomized study of paclitaxel (taxol) in patients with ovarian cancer previously treated with platinum [abstract]. Bristol-Myers Squibb (New Jersey) data on file. 1994
Kavanagh JJ, Kudelka AP, Edwards CL, et al. A randomized crossover trial of parenteral hydroxyurea vs high dose taxol in cisplatin/carboplatin resistant epithelial ovarian cancer [abstract 822]. Proc Am Soc Clin Oncol 1993; 12: 259
Anon. Taxol (paclitaxel) for injection concentrate. In advanced ovarian cancer after failure of first-line or subsequent chemotherapy. Princeton: Bristol-Myers Squibb Company, 1993
Sarosy G, Kohn E, Link C, et al. Taxol dose intensification (D.I.) in patients with recurrent ovarian cancer [abstract 716]. Proc Am Soc Clin Oncol 1992; 11: 226
Kohn E, Reed E, Link C, et al. A pilot study of taxol, cisplatin, cyclophosphamide, and G-CSF in newly diagnosed stage III/IV ovarian cancer [abstract 814]. Proc Am Soc Clin Oncol 1993; 12: 257
Reed E, Sarosy G, Kohn E, et al. Phase I study of paclitaxel (TAX) and cyclophosphamide (CTX) in recurrent adenocarcinoma of the ovary [abstract 1400]. Proc Am Assoc Cancer Res 1994; 35: 234
Behrens B, Copeland L, Balcerzak S, et al. A phase I taxol(T)/altretamine(A) doublet clinical trial in women with refractory ovarian cancer (OC) [abstract 868]. Proc Am Soc Clin Oncol 1994; 13: 271
Bruckner HW, Cagnoni PJ, Lee J-M, et al. A sequence of adriamycin and taxol infusions for refractory ovarian cancer [abstract 890]. Proc Am Soc Clin Oncol 1994; 13: 276
McGuire WP, Hoskins WJ, Brady MF, et al. A phase III trial comparing cisplatin/cytoxan (PC) and cisplatin/taxol (PT) in advanced ovarian cancer (AOC) [abstract 808]. Proc Am Soc Clin Oncol 1993; 12: 255
Ozols RF, Kilpatrick D, O’Dwyer P, et al. Phase I and pharmacokinetic study of taxol (T) and carboplatin (C) in previously untreated patients (pts) with advanced epithelial ovarian cancer (OC): a pilot study of the gynecologic oncology group [abstract 824]. Proc Am Soc Clin Oncol 1993; 12: 259
Gelmon K, Nabholtz JM, Bontenbal M, et al. Randomized trial of two doses of paclitaxel in metastatic breast cancer after failure of standard therapy [abstract 493]. Ann Oncol 1994; 5 Suppl. 5: 198
Holmes FA, Valero V, Theriault RL, et al. Phase II trial of taxol (T) in metastatic breast cancer (MBC) refractory to multiple prior treatments [abstract 178]. Proc Am Soc Clin Oncol 1993; 12: 94
Munzone E, Capri G, Demicheli R, et al. Activity of taxol (T) by 3 h infusion in breast cancer patients (pts) with clinical resistance to anthracyclines (A) [abstract 413]. Eur J Cancer 1993; 29A Suppl. 6: S79
Seidman AD, Barrett S, Hudis C, et al. Three hour taxol infusion as initial (I) and as salvage (S) chemotherapy of metastatic breast cancer (MBC) [abstract 65]. Proc Am Soc Clin Oncol 1994; 13: 66
Seidman A, Crown J, Reichman B, et al. Lack of clinical crossresistance of taxol (T) with anthracycline (A) in the treatment of metastatic breast cancer (MBC) [abstract 53]. Proc Am Soc Clin Oncol 1993; 12: 63
Fisherman J, McCabe M, Hillig M, et al. Phase I study of taxol plus doxorubicin plus granulocyte colony stimulating factor (G-CSF) in patients with metastatic breast cancer [abstract]. Abstract from the Second National Cancer Institute Workshop on Taxol and Taxus; 1992 Sep 23–24; Alexandria, 1992
Fisherman J, McCabe M, Hilig M, et al. Phase I study of taxol and doxorubicin (DOX) with G-CSF in previously untreated metastatic breast cancer (MBC) [abstract 54]. Proc Am Soc Clin Oncol 1992; 11: 57
Gianni L, Straneo M, Capri G, et al. Optimal dose and sequence finding study of paclitaxel (P) by 3 h infusion combined with bolus doxorubicin (D) in untreated metastatic breast cancer patients (pts) [abstract 97]. Proc Am Soc Clin Oncol 1994; 13: 74
Holmes FA, Valero V, Walters R, et al. Phase I study of doxorubicin followed by taxol (Tax) as initial chemotherapy for metastatic breast cancer (MBC) [abstract 321]. Abstract from the 18th International Congress of Chemotherapy; 1993 Jun 27–Jul 2; Stockholm, 1993
Holmes FA, Walters R, Valero V, et al. The M. D. Anderson experience with taxol in metastatic breast cancer [abstract]. Abstract from the Second National Cancer Institute Workshop on Taxol and Taxus; 1992 Sep 23–24; Alexandria, 1992
Sledge G, Sparano J, McCaskill-Stevens W, et al. Pilot trial of alternating taxol and adriamycin for metastatic breast cancer [abstract 85]. Proc Am Soc Clin Oncol 1993; 12: 71
Kennedy MJ, Armstrong D, Donehower R, et al. The hematologic toxicity of the taxol/cytoxan doublet is sequence-dependent [abstract 342]. Proc Am Soc Clin Oncol 1994; 13: 137
Tolcher A, Cowan K, Riley J, et al. Phase I study of paclitaxel (T) and cyclophosphamide (CTX) and G-CSF in metastatic breast cancer [abstract 93]. Proc Am Soc Clin Oncol 1994; 13:73
Gelmon KA, O’Reilly S, Plenderleith IH, et al. Bi-weekly paclitaxel and cisplatin in the treatment of metastatic breast cancer [abstract 87]. Proc Am Soc Clin Oncol 1994; 13: 71
Wasserheit C, Alter R, Speyer J, et al. Phase II trial of paclitaxel and cisplatin (DDP) in women with metastatic breast cancer [abstract 204]. Proc Am Soc Clin Oncol 1994; 13: 100
Hortobagyi GN, Holmes FA. Paclitaxel (taxol) therapy in breast cancer management. Cancer Invest 1994; 12 Suppl. 1:31–3
Kennedy MJ, Donehower RC, Sartorius SE, et al. Sequences of of taxol (T) and cyclophosphamide (C): a phase I and pharmacologic study in doxorubicin resistant metastatic breast cancer (DRMBC) [abstract 459]. Proc Am Soc Clin Oncol 1993; 12: 165
Klaassen U, Diergarten K, Dittrich CH, et al. Randomized trial of two doses of taxol in metastatic breast cancer: an interim analysis. Onkologie 1994; 17: 86–90
Hudis C, Seidman A, Baselga J, et al. Sequential high dose adjuvant doxorubicin (A), paclitaxel (T), and cyclophosphamide (C) with G-CSF (G) is feasible for women (pts) with resected breast cancer (BC) and ≥4(+) lymph nodes (LN) [abstract 62]. Proc Am Soc Clin Oncol 1994; 13: 65
Demetri GD, Berry D, Younger J, et al. Dose-intensified cyclophosphamide/doxorubicin (CD) followed by taxol (T) as adjuvant systemic chemotherapy for node-positive breast cancer (CALGB 9141): randomized comparison of two dose levels of G-CSF [abstract 63]. Proc Am Soc Clin Oncol 1994; 13: 65
Dieras V, Marty M, Morvan F, et al. A phase II randomized study of Taxol® versus mitomycin C in patients with advanced breast cancer: interim analysis [abstract 1683]. Proc Am Soc Clin Oncol 1994; 13: 112
Ajani JA, Ilson DH, Daugherty K, et al. Activity of taxol in patients with squamous cell carcinoma and adenocarcinoma of the esophagus. J Natl Cancer Inst 1994; 86: 1086–91
Einzig AI, Wiernik PH, Lipsitz S, et al. Phase II trial of taxol in patients with adenocarcinoma of the upper gastrointestinal tract (UGIT); The Eastern Cooperative Oncology Group (ECOG) results [abstract 566]. Proc Am Soc Clin Oncol 1993; 12: 194
Hutter H, Motzer R, Schwartz P, et al. Phase II trial of taxol in cisplatin-resistant germ cell tumor (GCT) patients (pts) [abstract 712]. Proc Am Soc Clin Oncol 1994; 13: 232
Vogelzang NJ, Herndon J, Clamon GH, et al. Paclitaxel (taxol) for malignant mesothelioma (MM): a phase II study of the Cancer and Leukemia Group B (CALGB 9234) [abstract 1382]. Proc Am Soc Clin Oncol 1994; 13: 405
Roth BJ, Dreicer R, Einhorn LH, et al. Paclitaxel in previously untreated, advanced transitional cell carcinoma of the urothelium: a phase II trial of the Eastern Cooperative Oncology Group (ECOG) [abstract 704]. Proc Am Soc Clin Oncol 1994; 13: 230
Roth BJ, Yeap BY, Wilding G, et al. Taxol in advanced, hormone-refractory carcinoma of the prostate. A phase II trial of the Eastern Cooperative Oncology Group. Cancer 1993; 72: 2457–60
Brown T, Tangen C, Fleming T, et al. A phase II trial of taxol and granulocyte colony stimulating factor (G-CSF) in patients with adenocarcinoma of the pancreas [abstract 592]. Proc Am Soc Clin Oncol 1993; 12: 200
Einzig AI, Gorowski E, Sasloff J, et al. Phase II trial of taxol in patients with metastatic renal cell carcinoma. Cancer Invest 1991; 9: 133–6
Hurwitz CA, Relling MV, Weitman SD, et al. Phase I trial of paclitaxel in children with refractory solid tumors: a Pediatric Oncology Group Study. J Clin Oncol 1993; 11: 2324–9
Donfrancesco A, Deb G, Sio L, et al. Phase I trial of a Q4D taxol regimen in pediatric patients with recurrent solid tumors [abstract 1463]. Proc Am Soc Clin Oncol 1994; 13: 426
Legha SS, Ring S, Papadopoulos N, et al. A phase II trial of taxol in metastatic melanoma. Cancer 1990; 65: 2478–81
Einzig AI, Hochster H, Wiernik PH, et al. A phase II study of taxol in patients with malignant melanoma. Invest New Drugs 1991; 9: 59–64
Ettinger DS. Overview of paclitaxel (Taxol®) in advanced lung cancer. Semin Oncol 1993; 20 Suppl. 3: 46–9
Eisenhauer EA. Taxol in advanced non-small-cell lung cancer: plus ça change?. J Natl Cancer Inst 1993; 85: 346–7
Chang AY, Kim K, Glick J, et al. Phase II study of taxol, merbarone, and piroxantrone in stage IV non-small-cell lung cancer: the Eastern Cooperative Oncology Group results. J Natl Cancer Inst 1993; 85: 388–94
Murphy WK, Fossella FV, Winn RJ, et al. Phase II study of taxol in patients with untreated advanced non-small-cell lung cancer. J Natl Cancer Inst 1993; 85: 384–8
Murphy WK, Winn RJ, Huber M, et al. Phase II study of taxol (T) in patients (pts) with non-small cell lung cancer (NSCLC) who have failed platinum (P) containing chemotherapy (CTX) [abstract 1224]. Proc Am Soc Clin Oncol 1994; 13: 363
Ruckdeschel J, Wagner Jr H, Williams C, et al. Second-line chemotherapy for resistant, metastatic, non-small cell lung cancer (NSCLC): the role of taxol (TAX) [abstract 1200]. Proc Am Soc Clin Oncol 1994; 13: 357
Israel VK, Zaretsky S, Natale RB. Phase I/II trial of combination carboplatin and taxol in advanced non-small cell lung cancer (NSCLC) [abstract 1175]. Proc Am Soc Clin Oncol 1994; 13: 351
Langer CJ, Leighton J, Comis R, et al. Taxol and carboplatin (CBDCA) in combination in stage IV and IIIB non small-cell lung cancer (NSCLC): a phase II trial [abstract 1122]. Proc Am Soc Clin Oncol 1994; 13: 338
Paul DM, Johnson DH, Hande KR, et al. Carboplatin (C) and taxol (T): a well tolerated regimen for advanced non-small cell lung cancer (NSCLC) [abstract 1181]. Proc Am Soc Clin Oncol 1994; 13: 352
Hainsworth JD, Miller P, Menchise A, et al. Treatment of locally advanced non-small cell lung cancer (NSCLC) with taxol (1-hour infusion, cisplatin, etoposide, and radiation therapy (RT): a phase II trial [abstract 1172]. Proc Am Soc Clin Oncol 1994; 13: 350
Klastersky J, Schlier JP. Paclitaxel (P) plus cisplatin (C) in non small cell lung cancer: a dose finding study [abstract 1169]. Proc Am Soc Clin Oncol 1994; 13: 349
Kirschling RJ, Jung SH, Jett JR, et al. A phase II trial of taxol and GCSF in previously untreated patients with extensive stage small cell lung cancer (SCC) [abstract 1076]. Proc Am Soc Clin Oncol 1994; 13: 326
Hainsworth JD, Erland J, Peters M, et al. Treatment of small cell lung cancer (SCLC) with taxol (1-hour infusion), carboplatin, and etoposide: phase II study of outpatient regimen [abstract 1171]. Proc Am Soc Clin Oncol 1994; 13: 350
Thornton D, Singh K, Putz B, et al. A phase II trial of taxol in squamous cell carcinoma of the head and neck [abstract 933]. Proc Am Soc Clin Oncol 1994; 13: 288
Forastiere AA. Use of paclitaxel (TAXOL®) in squamous cell carcinoma of the head and neck. Semin Oncol 1993; 20 Suppl. 3: 56–60
Younes A, Sarris A, McLaughlin P, et al. Phase II trial of taxol given as a 3-hour infusion every 3 weeks in relapsed non-Hodgkin’s lymphoma (NHL) [abstract 1267]. Proc Am Soc Clin Oncol 1994; 13: 374
Dimopoulos M, Arbuck S, Weber D, et al. Primary paclitaxel (taxol) therapy for previously untreated multiple myeloma [abstract 1394]. Proc Am Soc Clin Oncol 1994; 13: 409
Feldman E, Seiter K, Helson L, et al. Phase I evaluation of a short infusion every 4 day schedule of taxol in refractory acute leukemia [abstract 1022]. Proc Am Soc Clin Oncol 1994; 13: 311
Strauman JJ. Symptom distress in patients receiving phase I chemotherapy with taxol. Oncology Nursing Forum 1986; 13: 40–3
Bicher A, Sarosy G, Kohn E, et al. Age does not influence taxol dose intensity in recurrent carcinoma of the ovary. Cancer 1993; 71 Suppl.: 594–600
Zaheer W, Lichtman SM, DeMarco L, et al. The use of taxol in elderly patients [abstract 1518]. Proc Am Soc Clin Oncol 1994; 13: 441
Peereboom DM, Donehower RC, Eisenhauer EA, et al. Successful re-treatment with taxol after major hypersensitivity reactions. J Clin Oncol 1993; 11: 885–90
Bissett D, Kaye SB. Taxol and taxotere — current status and future prospects. Eur J Cancer 1993; 29A: 1228–31
Weiss RB, Donehower RC, Wiernik PH, et al. Hypersensitivity reactions from taxol. J Clin Oncol 1990; 8: 1263–8
Trimble EL, Adams JD, Vena D, et al. Taxol in patients with platinum-refractory ovarian cancer [abstract 829]. Proc Am Soc Clin Oncol 1993; 12: 261
Sulkes A, Belier U, Peretz T, et al. Taxol: initial Israeli experience with a novel anticancer agent. Isr J Med Sci 1994; 30: 70–8
Pagani O, Sessa C, Goldhirsch A, et al. Taxol (T) and cyclophosphamide (C) in patients (pts) with advanced breast cancer (ABC): a dose-finding study with the addition of G-CSF [abstract 45]. Proc Am Soc Clin Oncol 1994; 13:61
Sledge GW, Robert N, Goldstein LJ, et al. Phase I trial of adriamycin (A) + taxol (T) in metastatic breast cancer (MBC) [abstract 42.1]. Eur J Cancer 1993; 29A Suppl. 6: S81
Rosenthal CJ, Ibrahim A, Rambhia H, et al. Paclitaxel (taxol)-mitoxantrone salvage therapy in metastatic carcinoma of the breast-phase I/II study [abstract 172], Proc Am Soc Clin Oncol 1994; 13: 92
Jerian SM, Sarosy GA, Link Jr CJ, et al. Incapacitating autonomic neuropathy precipitated by taxol. Gynecol Oncol 1993; 51: 277–80
Capri G, Munzone E, Tarenzi E, et al. Optic nerve disturbances: a new form of paclitaxel neurotoxicity. J Natl Cancer Inst 1994; 86: 1099–101
Schalhorn B. Paclitaxel (taxol) — a cytostatic with a novel mechanism of action. Med Klin 1993; 88 Suppl. II: 4–6
Arbuck SG, Adams J, Strauss H, et al. Areassessment of cardiac toxicity associated with taxol [abstract]. Abstract from the Second National Cancer Institute Workshop on Taxol and Taxus; 1992 Sep 23–24; Alexandria. 1992.
Rowinsky EK, Eisenhauer EA, Chaudhry V, et al. Clinical toxicities encountered with paclitaxel (Taxol®). Semin Oncol 1993; 20 Suppl. 3: 1–15
Rowinsky EK, McGuire WP, Guarnieri T, et al. Cardiac disturbances during the administration of taxol. J Clin Oncol 1991; 9: 1704–12
Hurwitz C, Relling M, Ragab A, et al. Phase I trial of taxol in children with refractory solid tumors: a pediatric oncology group study [abstract 1410]. J Clin Oncol 1993; 12: 412
Biadi O, Mengozzi G, Gherarducci G, et al. Evaluation of taxol cardiotoxicity in metastatic breast cancer. Ann N Y Acad Sci 1993; 698: 403–5
Gibbs H, Ewer M, Holmes F, et al. Cardiac monitoring during administration of taxol-doxorubicin chemotherapy in patients with metastatic breast cancer: a preliminary report [abstract 170]. Proc Am Soc Clin Oncol 1992; 11: 86
Raghavan VT, Bloomer WD, Merkel DE. Taxol and radiation recall dermatitis. Lancet 1993; 341: 1354
Ajani JA, Dodd LG, Daugherty K, et al. Taxol-induced soft-tissue injury secondary to extravasation: characterization by histopathology and clinical course. J Natl Cancer Inst 1994; 86: 51–3
Marsoni S, Ungerleider RS, Hurson SB, et al. Tolerance to antineoplastic agents in children and adults. Cancer Treat Rep 1985; 69: 1263–9
Author information
Authors and Affiliations
Additional information
Various sections of the manuscript reviewed by: B. Bissett, CRC Department of Medical Oncology, University of Glasgow, Beatson Oncology Centre, Western Infirmary, Glasgow, Scotland; C. Bottex-Gauthier, Departement de Biologie Cellulaire, Centre de Recherches du Service de Sante des Armees Emile Parde, Minstere de la Defense, La Tronche, France; A.I. Einzig, Clinical Oncology Program, Albert Einstein College of Medicine, Bronx, New York, USA; A.R. Hanauske, Abteilung für Hämatologie und Onkologie, Medizinische Klinik und Poliklinik der Technischen Universität München, Munich, Germany; C.A. Hurwitz, Maine Children’s Cancer Program, Portland, Maine, USA; T. Ide, Department of Cellular and Molecular Biology, Hiroshima University School of Medicine, Hiroshima City, Hiroshima, Japan; D.R. Kohler, Pharmacy Department, Warren G. Magnuson Clinical Center, National Institutes of Health, Bethesda, Maryland, USA; E.K. Rowinsky, Division of Pharmacology and Experimental Therapeutics, Johns Hopkins Oncology Center, Baltimore, Maryland, USA; N. Saijo, Pharmacology Division and Medical Oncology, National Cancer Center, Research Institute and Hospital, Tokyo, Japan.
Rights and permissions
About this article
Cite this article
Spencer, C.M., Faulds, D. Paclitaxel. Drugs 48, 794–847 (1994). https://doi.org/10.2165/00003495-199448050-00009
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-199448050-00009