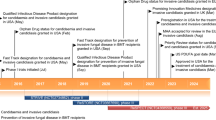
Abstract
Intravenous micafungin (Mycamine®; Fungard®), an echinocandin, is approved in the EU for the treatment of adult (aged ≥16 years) and paediatric patients with invasive candidiasis and for the treatment of adult patients with oesophageal candidiasis. It is also approved in the EU as prophylactic treatment to prevent Candida infections in adult and paediatric patients undergoing haematopoietic stem cell transplant (HSCT) or patients who are expected to have neutropenia for ≥10 days. This article reviews the therapeutic use of micafungin for the treatment and prophylaxis of Candida infections in adult and paediatric patients, focusing on approved indications in Europe, and briefly discusses the pharmacology of the drug.
Micafungin shows very good in vitro activity against clinically relevant isolates of Candida spp., with a low propensity to be associated with the emergence of resistant isolates. The drug has a convenient once-daily dosage regimen and is associated with relatively few drug-drug interactions. In large, multinational trials in adult and/or paediatric patients with invasive candidiasis, micafungin was noninferior to intravenous caspofungin or liposomal amphotericin B. In similarly designed trials in adult patients with oesophageal candidiasis, treatment with micafungin was noninferior to that with intravenous fluconazole or caspofungin. As prophylactic treatment in adult and paediatric patients who had undergone HSCT, micafungin was superior to fluconazole therapy and noninferior to oral itraconazole in large, multicentre trials. Micafungin was generally well tolerated by participants in these clinical trials, given the severe morbidity of the underlying conditions of patients, with a similar tolerability profile to caspofungin and, in general, to fluconazole. It was better tolerated than liposomal amphotericin B or oral itraconazole. Thus, micafungin is a valuable first-line or alternative option to other antifungal agents for the management of candidaemia and invasive candidiasis in adult and paediatric patients, including neonates, and as prophylaxis against fungal infections in patients undergoing HSCT.








Similar content being viewed by others
References
Pappas PG. Micafungin for candidiasis. Mycoses 2012; 55 Suppl. 1: 8–12
Playford EG, Lipman J, Sorrell TC. Management of invasive candidiasis in the intensive care unit. Drugs 2010; 70(7): 823–39
Arendrup MC. Epidemiology of invasive candidiasis. Curr Opin Crit Care 2010; 16(5): 445–52
Glöckner A. Treatment and prophylaxis of invasive candidiasis with anidulafungin, caspofungin and micafungin: review of the literature. Eur J Med Res 2011 Apr 28; 16(4): 167–79
Tragiannidis A, Dokos C, Lehrnbecher T, et al. Antifungal chemoprophylaxis in children and adolescents with haematological malignancies and following allogeneic haematopoietic stem cell transplantation: review of the literature and options for clinical practice. Drugs 2012; 72(5): 685–704
Maertens J, Marchetti O, Herbrecht R, et al. European guidelines for antifungal management in leukemia and hematopoietic stem cell transplant recipients: summary of the ECIL 3 — 2009 update. Bone Marrow Transplant 2011; 46(5): 709–18
Kullberg BJ, Verweij PE, Akova M, et al. European expert opinion on the management of invasive candidiasis in adults. Clin Microbiol Infect 2011; 17 Suppl. 5: 1–12
Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 48(5): 503–35
Cuenca-Estrella M, Verweij PE, Arendrup MC, et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: diagnostic procedures. Clin Microbiol Infect. Epub 2012; doi: 10.1111/1469-0691.12038
Ullmann AJ, Cornely OA, Donnelly JP, et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: developing European guidelines in clinical microbiology and infectious diseases. Clin Microbiol Infect. Epub 2012. doi: 10.1111/1469-0691.12041
Cornely OA, Bassetti M, Calandra T, et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. Epub 2012. doi: 10.1111/1469-0691.12039
Hope WW, Castagnola E, Groll AH, et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: prevention and management of invasive infections in neonates and children caused by Candida spp. Clin Microbiol Infect. Epub 2012. doi: 10.1111/1469-0691.12040
Ullmann AJ, Akova M, Herbrecht R, et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: adults with heamatological malignancies and after haematopoietic stem cell transplantation (HCT). Clin Microbiol Infect. Epub 2012. doi: 10.1111/1469-0691.12037
Lortholary O, Petrikkos G, Akova M, et al. ESCMID guideline for the diagnosis and management of Candida diseases 2012: patients with HIV infection or AIDS. Clin Microbiol Infect. Epub 2012. doi: 10.1111/1469-0691.12042
Andes DR, Safdar N, Baddley JW, et al. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin Infect Dis 2012; 54(8): 1110–22
Cross SA, Scott LJ. Micafungin: a review of its use in adults for the treatment of invasive and oesophageal candidiasis, and as prophylaxis against Candida infections. Drugs 2008; 68(15): 2225–55
Carter NJ, Keating GM. Micafungin: a review of its use in the prophylaxis and treatment of invasive Candida infections in pediatric patients. Paediatr Drugs 2009; 11(4): 271–91
Chandrasekar PH, Sobel JD. Micafungin: a new echinocandin. Clin Infect Dis 2006; 48(8): 1171–8
European Committee on Antimicrobial Susceptibility Testing. Antifungal agents: breakpoint tables for the interpretation of MICs version 4.1 [online]. Available from URL: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Antifungal_breakpoints_v_4.1.pdf [Accessed 2012 Jul 2]
Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing for yeasts; informational supplement M27-S3. Wayne (PA): Clinical and Laboratory Standards Institute, 2008
Simjee S, Silley P, Werling HO, et al. Potential confusion regarding the term resistance in epidemiological surveys [letter]. J Antimicrob Chemother 2008; 61(1): 228–9
Kahlmeter G, Brown DFJ, Goldstein FW, et al. European harmonisation of MIC breakpoints for antimicrobial susceptibility testing of bacteria. J Antimicrob Chemother 2003; 52(2): 145–8
Pfaller MA, Boyken L, Hollis RJ, et al. Wild-type MIC distributions and epidemiological cutoff values for echinocandins and Candida spp. J Clin Microbiol 2010; 48(1): 52–6
Pfaller M, Boyken L, Hollis R, et al. Use of epidemiological cutoff values to examine 9-year trends in susceptibility of Candida species to anidulafungin, caspofungin, and micafungin. J Clin Microbiol 2011; 49(2): 624–9
Pfaller MA, Diekema DJ, Andes D, et al. Clinical breakpoints for the echinocandins and Candida revisited: integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resist Updates 2011; 14(3): 164–76
Pfaller MA, Castanheira M, Diekema DJ, et al. Comparison of European Committee on Antimicrobial Susceptibility Testing (EUCAST) and Etest methods with the CLSI broth microdilution method for echinocandin susceptibility testing against Candida species. J Clin Microbiol 2010; 48(5): 1592–9
Pfaller MA, Boyken L, Hollis RJ, et al. In vitro susceptibility of invasive isolates of Candida spp. to anidulafungin, caspofungin, and micafungin: six years of global surveillance [published erratum appears in J Clin Microbiol 2008; 46 (9): 3184–5]. J Clin Microbiol 2008; 46(1): 150–6
Shields RK, Nguyen MH, Press EG, et al. The presence of an FKS mutation rather than MIC is an independent risk factor for failure of echinocandin therapy among patients with invasive Candidiasis due to Candida glabrata. Antimicrob Agents Chemother 2012; 56(9): 4862–9
Kuhn DM, George T, Chandra J, et al. Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother 2002; 46(6): 1773–80
Seidler M, Salvenmoser S, Muller FM. In vitro effects of micafungin against Candida biofilms on polystyrene and central venous catheter sections. Int J Antimicrob Agents 2006; 28(6): 568–73
Hanadate T, Wakasugi M, Sogabe K, et al. Evaluation of the safety and efficacy of micafungin in Japanese patients with deep mycosis: a post-marketing survey report [published erratum appears in J Infect Chemother 2011; 17 (5): 633 Note: Dosage error in article text]. J Infect Chemother 2011; 17(5): 622–32
Pappas PG, Rotstein CM, Betts RF, et al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin Infect Dis 2007; 45(7): 883–93
Kuse ER, Chetchotisakd P, da Cunha CA, et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: a phase III randomised double-blind trial. Lancet 2007; 369(9572): 1519–27
Andes D, Ambrose PG, Hammel JP, et al. Use of pharmacokinetic-pharmacodynamic analyses to optimize therapy with the systemic antifungal micafungin for invasive candidiasis or candidemia. Antimicrob Agents Chemother 2011; 55(5): 2113–21
Undre N, Stevenson P, Baraldi E. Pharmacokinetics of micafungin in HIV positive patients with confirmed esophageal candidiasis. Eur J Drug Metab Pharmacokinet 2012; 37(1): 31–8
Seibel NL, Schwartz C, Arrieta A, et al. Safety, tolerability, and pharmacokinetics of micafungin (FK463) in febrile neutropenic pediatric patients. Antimicrob Agents Chemother 2005; 49(8): 3317–24
Undre NA, Stevenson P, Freire A, et al. Pharmacokinetics of micafungin in pediatric patients with invasive candidiasis and candidemia. Pediatr Infect Dis J 2012; 31(6): 630–2
Kuse E, Demeyer I, Stevenson P, et al. Pharmacokinetics of micafungin in adult patients with invasive candidiasis and candidemia [poster no. P022]. 28th International Symposium on Intensive Care and Emergency Medicine; 2008 Mar 18–21; Brussels
Undre N, Pretorius B, Botha FCJ, et al. Pharmacokinetics (PK) of micafungin in subjects with severe hepatic dysfunction (SHD) [abstract plus poster no. A1-594]. 49th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2009 Sep 12–15; San Francisco (CA)
Hebert MF, Smith HE, Marbury TC, et al. Pharmacokinetics of micafungin in healthy volunteers, volunteers with moderate liver disease, and volunteers with renal dysfunction. J Clin Pharmacol 2005; 45(10): 1145–52
Heresi GP, Gerstmann DR, Reed MD, et al. The pharmacokinetics and safety of micafungin, a novel echinocandin, in premature infants. Pediatr Infect Dis J 2006; 25(12): 1110–5
Mycamine 50 mg powder for solution for infusion: summary of product characteristics [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000734/WC500031075.pdf [Accessed 2012 Jun 24
Tabata K, Katashima M, Kawamura A, et al. Linear pharmacokinetics of micafungin and its active metabolites in Japanese pediatric patients with fungal infections. Biol Pharm Bull 2006; 29(8): 1706–11
Tabata K, Katashima M, Kawamura A, et al. Pharmacokinetics-pharmacodynamics of micafungin in Japanese patients with deep-seated mycosis. Eur J Drug Metabol Pharmacokinet 2006; 31(2): 123–8
Benjamin DK, Jr., Smith PB, Arrieta A, et al. Safety and pharmacokinetics of repeat-dose micafungin in young infants. Clin Pharmacol Ther 2010; 87(1): 93–9
Smith PB, Walsh TJ, Hope W, et al. Pharmacokinetics of an elevated dosage of micafungin in premature neonates. Pediatr Infect Dis J 2009; 28(5): 412–5
Nicasio AM, Tessier PR, Nicolau DP, et al. Bronchopulmonary disposition of micafungin in healthy adult volunteers. Antimicrob Agents Chemother 2009; 53(3): 1218–20
Yamada N, Kumada K, Kishino S, et al. Distribution of micafungin in the tissue fluids of patients with invasive fungal infections. J Infect Chemother 2011; 17(5): 731–4
Walsh TJ, Goutelle S, Jelliffe RW, et al. Intrapulmonary pharmacokinetics and pharmacodynamics of micafungin in adult lung transplant patients. Antimicrob Agents Chemother 2010; 54(8): 3451–9
Mochizuki K, Suemori S, Udo K, et al. Intraocular penetration of micafungin in patient with Candida albicans endophthalmitis. J Ocul Pharmacol Ther 2011; 27(5): 531–3
Ohge H, Shimazutsu K, Shimizu Y, et al. Appropriate dosage of micafungin for Candida peritonitis [abstract no. A1-032]. 50th Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); 2010 Sep 12–15; Boston (MA)
Okugawa S, Ota Y, Tatsuno K, et al. A case of invasive central nervous system aspergillosis treated with micafungin with monitoring of micafungin concentrations in the cerebrospinal fluid. Scand J Infect Dis 2007; 39(4): 344–6
Lat A, Thompson GR, Rinaldi MG, et al. Micafungin concentrations from brain tissue and pancreatic pseudocyst fluid. Antimicrob Agents Chemother 2010; 54(2): 943–4
Mochizuki N, Matsumoto K, Ohno K, et al. Effects of hepatic CYP3A4 activity on disposition of micafungin in liver transplant recipients with markedly small-for-size grafts. Transplant Proceed 2006; 38(10): 3649–50
Hirata K, Aoyama T, Matsumoto Y, et al. Pharmacokinetics of antifungal agent micafungin in critically ill patients receiving continuous hemodialysis filtration. Yakugaku Zasshi 2007; 127(5): 897–901
Kishino S, Ohno K, Shimamura T, et al. Optimal prophylactic dosage and disposition of micafungin in living donor liver recipients. Clin Transplant 2004; 18(6): 676–80
Sakaeda T, Iwaki K, Kakumoto M, et al. Effect of micafungin on cytochrome P450 3A4 and multidrug resistance protein 1 activities, and its comparison with azole antifungal drugs. J Pharm Pharmacol 2005; 57(6): 759–64
de Wet NT, Bester AJ, Viljoen JJ, et al. A randomized, double blind, comparative trial of micafungin (FK463) vs. fluconazole for the treatment of oesophageal candidiasis. Aliment Pharmacol Ther 2005; 21(7): 899–907
Buell D, Kovanda L, Drake T, et al. Alternate day dosing of micafungin in the treatment of esophageal candidiasis [abstract no. M-719]. 45th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2005 Dec 16–19; Washington, DC
European Medicines Agency. Assessment report for mycamine [online]. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/000734/WC500031079.pdf [Accessed 2012 June 24
Chen Q, Lin MH, Chen ML, et al. Efficacy and safety of micafungin for invasive Candida infections: a meta-analysis of randomized controlled trials. Chinese Med J 2012; 125(2): 345–51
Queiroz-Telles F, Berezin E, Leverger G, et al. Micafungin versus liposomal amphotericin B for pediatric patients with invasive candidiasis: substudy of a randomized double-blind trial. Pediatr Infect Dis J 2008; 27(9): 820–6
de Wet N, Llanos-Cuentas A, Suleiman J, et al. A randomized, double-blind, parallel-group, dose-response study of micafungin compared with fluconazole for the treatment of esophageal candidiasis in HIV-positive patients. Clin Infect Dis 2004; 39(6): 842–9
Ostrosky-Zeichner L, Kontoyiannis D, Raffalli J, et al. International, open-label, noncomparative, clinical trial of micafungin alone and in combination for treatment of newly diagnosed and refractory candidemia. Eur J Clin Microbiol Infect Dis 2005; 24(10): 654–61
Yoshida M, Tamura K, Imamura M, et al. Efficacy and safety of micafungin as an empirical antifungal therapy for suspected fungal infection in neutropenic patients with hematological disorders. Ann Hematol 2012; 91(3): 449–57
Yamaguchi M, Kurokawa T, Ishiyama K, et al. Efficacy and safety of micafungin as an empirical therapy for invasive fungal infections in patients with hematologic disorders: a multicenter, prospective study. Ann Hematol 2011; 90(10): 1209–17
Pettengell K, Mynhardt J, Kluyts T, et al. Successful treatment of oesophageal candidiasis by micafungin: a novel systemic antifungal agent. Aliment Pharmacol Ther 2004; 20(4): 475–81
Tamura K, Urabe A, Yoshida M, et al. Efficacy and safety of micafungin, an echinocandin antifungal agent, on invasive fungal infections in patients with hematological disorders. Leukemia Lymphoma 2009; 50(1): 92–100
Kubiak DW, Bryar JM, McDonnell AM, et al. Evaluation of caspofungin ormicafungin as empiric antifungal therapy in adult patients with persistent febrile neutropenia: a retrospective, observational, sequential cohort analysis. Clin Ther 2010; 32(4): 637–48
Dupont BF, Lortholary O, Ostrosky-Zeichner L, et al. Treatment of candidemia and invasive candidiasis in the intensive care unit: post hoc analysis of a randomized, controlled trial comparing micafungin and liposomal amphotericin B. Crit Care 2009; 13(5): R159
Nucci M, Anaissie E, Betts RF, et al. Early removal of central venous catheter in patients with candidaemia does not improve outcome: analysis of 482 patients from 2 randomized clinical trials. Clin Infect Dis 2010; 51(3): 295–303
Shorr AF, Wu C, Kothari S. Outcomes with micafungin in patients with candidaemia or invasive candidiasis due to Candida glabrata and Candida krusei. J Antimicrob Chemother 2011; 66(2): 375–80
Cornely OA, Marty FM, Stucker F, et al. Efficacy and safety of micafungin for treatment of serious Candida infections in patients with or without malignant disease. Mycoses 2011; 54(6): e838–47
van Burik JA, Ratanatharathorn V, Stepan DE, et al. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis 2004; 39(10): 1407–16
Huang X, Chen H, Han M, et al. Multicenter, randomized, open-label study comparing the efficacy and safety of micafungin versus itraconazole for prophylaxis of invasive fungal infections in patients undergoing hematopoietic stem cell transplant. Biol Blood Marrow Transplant. Epub 2012 Mar 30
Hiramatsu Y, Maeda Y, Fujii N, et al. Use of micafungin versus fluconazole for antifungal prophylaxis in neutropenic patients receiving hematopoietic stem cell transplantation. Int J Hematol 2008; 88(5): 588–95
Kusuki S, Hashii Y, Yoshida H, et al. Antifungal prophylaxis with micafungin in patients treated for childhood cancer. Pediatr Blood Cancer 2009; 53(4): 605–9
Cornely OA, Pappas PG, Young JA, et al. Accumulated safety data of micafungin in therapy and prophylaxis in fungal diseases. Expert Opin Drug Saf 2011; 10(2): 171–83
Arrieta AC, Maddison P, Groll AH. Safety of micafungin in pediatric clinical trials. Pediatr Infect Dis J. 2011; 30(6): e97–e102
Sirohi B, Powles RL, Chopra R, et al. A study to determine the safety profile and maximum tolerated dose of micafungin (FK463) in patients undergoing haematopoietic stem cell transplantation. Bone Marrow Transplant 2006; 38(1): 47–51
Hiemenz J, Cagnoni P, Simpson D, et al. Pharmacokinetic and maximum tolerated dose study of micafungin in combination with fluconazole versus fluconazole alone for prophylaxis of fungal infections in adult patients undergoing a bone marrow or peripheral stem cell transplant. Antimicrob Agents Chemother 2005; 49(4): 1331–6
Pullaiah B, Hatch B, Shannon D, et al. Hepatic safety of long-term exposure to caspofungin or micafungin among patients at Duke University Medical Center [poster no. M-1052]. 50th Interscience Conference on Antimicrobial Agents and Chemotherapy; 2010 Sep 12–15; Boston (MA)
Tortorano AM, Kibbler C, Peman J, et al. Candidaemia in Europe: epidemiology and resistance. Int J Antimicrob Agents 2006; 27(5): 359–66
Mehta PA, Vinks AA, Filipovich A, et al. Alternate-day micafungin antifungal prophylaxis in pediatric patients undergoing hematopoietic stem cell transplantation: a pharmacokinetic study. Biol Blood Marrow Transplant 2010; 16(10): 1458–62
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by:M. Bassetti, Clinica Malattie Infective, Azienda Osperdaliera Universitania San Martino, Genova, Italy; B. T. Fisher, Division of Infectious Diseases, Children’s Hospital of Philadelphia, Philadelphia, PA, USA; A. H. Groll, Infectious Diseases Research Program, Center for Bone Marrow Transplantation and Department of Pediatric Hematology/Oncology, University Children’s Hospital Münster, Albert-Schweitzer Campus-1, Münster, Germany; M. Kalin, Department of Medicine Infektionskliniken, Karolinska Institute, Stockholm, Sweden; B. M. Lomaestro, Albany Medical Center Hospital Pharmacy, Albany, NY, USA; T. Rogers, St James’s Hospital, Trinity College Dublin, Department of Clinical Microbiology, Dublin, Ireland.
Data Selection
Sources: Medical literature (including published and unpublished data) on ‘micafungin’ was identified by searching databases (including MEDLINE and EMBASE) for articles published since 1996, bibliographies from published literature, clinical trial registries/databases and websites (including those of regional regulatory agencies and the manufacturer). Additional information (including contributory unpublished data) was also requested from the company developing the drug.
Search strategy: MEDLINE and EMBASE search terms were ‘micafungin’ and (‘candidiasis’ or ‘candidaemia’ or ‘candidemia’). Searches were last updated 8 October 2012.
Selection: Studies in patients with invasive or oesophageal candidiasis or candidaemia who received micafungin treatment, or in haematopoietic stem cell transplant recipients who received micafungin as prophylaxis. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Micafungin, echinocandin, antifungal, candidaemia, invasive candidiasis, oesophageal candidiasis, prophylaxis, pharmacodynamics, pharmacokinetics, therapeutic use.
Rights and permissions
About this article
Cite this article
Scott, L.J. Micafungin. Drugs 72, 2141–2165 (2012). https://doi.org/10.2165/11209970-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11209970-000000000-00000