Summary
Abstract
Insulin glulisine (Apidra®) is a human insulin analogue approved for the improvement of glycaemic control in adults, adolescents and children with diabetes mellitus. It has similar binding properties, and is associated with a faster onset but similar level of glucose disposal, to regular human insulin (RHI). Insulin glulisine and insulin lispro have similar effects on glucose levels.
Insulin glulisine is effective when compared to other short- and rapid-acting insulins, demonstrating either noninferiority, no significant difference, or superiority in primary endpoints in studies involving patients with type 1 and type 2 diabetes. It is more effective and has a faster onset and shorter duration of activity than RHI. Insulin glulisine is as effective as insulin lispro in patients with type 1 diabetes; however, there is a need for further, well designed head-to-head comparisons with insulin lispro in patients with type 2 diabetes and with insulin aspart in patients with type 1 or type 2 diabetes to fully establish the place of insulin glulisine in the management of diabetes. Insulin glulisine has a flexible administration period, as it can be administered immediately before or after meals. Hypoglycaemia, a common risk with insulins, occurs at a similar rate among recipients of insulin glulisine to that seen with other insulins. Thus, insulin glulisine is an effective and well tolerated option for the treatment of patients with type 1 and type 2 diabetes.
Pharmacological Properties
Human insulin was altered to form insulin glulisine by replacing asparagine with lysine at position B3 and lysine with glutamic acid at position B29. The majority of in vitro and in vivo studies have shown that the binding of insulin glulisine to the insulin receptor (to therapeutic effect) and to the insulin-like growth factor-1 receptor (to mitogenic effect) is similar to that of RHI.
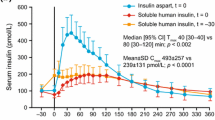
Insulin glulisine is associated with a faster glucose-lowering effect than RHI, but shows a similar level of glucose disposal overall, in patients with type 1 or type 2 diabetes. Insulin glulisine and insulin lispro appear similar with regard to speed and extent of glucose lowering in patients with type 1 or type 2 diabetes. In clinical trials in patients with type 1 or type 2 diabetes, insulin glulisine was associated with preprandial blood glucose levels that were at least as low as comparator agents, and on occasion significantly lower than those seen with RHI or (with basal insulin glargine) premixed insulin. Body mass index (BMI) does not appear to have an effect on blood glucose control in volunteers.
The majority of clinical trials reported (where stated) no significant difference between insulin glulisine and comparator agents in terms of change in body-weight. Insulin glulisine appears to be associated with a closer approximation of normal microvascular blood flow and endothelial function than RHI, and end-organ metabolic effects associated with the administration of insulin glulisine, insulin lispro and RHI appear to be similar.
Insulin glulisine appears to have a similar total systemic availability to RHI, but with faster absorption, in patients with type 1 and type 2 diabetes, whereas it does not differ from insulin lispro with regard to these parameters in these patient populations. Patients with type 1 and type 2 diabetes had a maximum serum insulin glulisine concentration (Cmax) of 120 and 92 μU/mL, a time to Cmax of 51 and 83 minutes, and an area under the insulin glulisine concentration-time curve from time zero to endpoint of 16120 and 18408 μU · min/mL. Timing of administration (with regard to meal) and injection site did not appear to have significant effects on pharmacokinetic parameters.
Insulin glulisine, administered by subcutaneous injection, has an approximate absolute bioavailability of 70% and is associated with low plasma protein binding. After intravenous administration, the volume of distribution and elimination of insulin glulisine appears to be similar to those of RHI, but after subcutaneous administration the elimination is more rapid than with RHI. Insulin glulisine has a total clearance of 927 mL/min after intravenous administration and its metabolism is unlikely to differ from that of RHI.
Age, BMI and ethnicity do not appear to have any effect on the pharmacokinetic parameters of insulin glulisine. A reduction in the dosage of insulin may be required in the presence of renal impairment. The pharmacokinetic effects of insulin glulisine have not been investigated in patients with impaired liver function.
Therapeutic Efficacy
Several well designed trials have investigated the efficacy of insulin glulisine (with and without basal insulin) versus comparator agents (with and without basal insulin) in patients with type 1 and type 2 diabetes. In patients with type 1 diabetes, insulin glulisine was noninferior to insulin lispro (in both adult and paediatric patients) and to RHI (in adult patients). In adult patients with type 2 diabetes, insulin glulisine was noninferior (and superior in one study) to RHI and (with basal insulin glargine) more effective than premixed insulin. The primary endpoint in most clinical trials, where stated, was the change in glycosylated haemoglobin (HbA1c) levels from baseline to the end of the study period.
There were no significant differences between pre- and postprandial administration, or between pre-breakfast and pre-main meal administration, among patients with type 2 diabetes. Postprandial insulin glulisine was noninferior to preprandial in the primary endpoint in patients with type 1 diabetes; however, within this wide noninferiority margin (0.4% difference in change in HbA1c), preprandial insulin glulisine was shown to be significantly superior to postprandial administration.
When administered using continuous subcutaneous insulin infusion (CSII), insulin glulisine appeared to be as effective as insulin aspart in patients with type 1 diabetes. A basal-bolus regimen of insulin glargine and insulin glulisine was more effective than a sliding-scale regimen of RHI in hospitalized patients with type 2 diabetes.
Tolerability
Insulin glulisine was well tolerated in clinical studies of patients with type 1 and type 2 diabetes. Hypoglycaemia generally occurred at a similar rate and incidence among insulin glulisine versus comparator insulin recipients among adult and paediatric patients with type 1 diabetes and in adult patients with type 2 diabetes. No significant difference was reported between insulin glulisine and comparator agents in the overall incidence of adverse events, the incidence of serious adverse events or discontinuation due to adverse events in clinical trials. Discontinuation due to adverse events was rare, as were adverse events other than hypoglycaemia. No significant laboratory test differences from baseline were reported in insulin glulisine recipients.
Patients with type 1 diabetes receiving insulin glulisine showed a slight decrease in cross-reactive insulin antibody levels at endpoint versus baseline; no such difference was reported among patients with type 2 diabetes.
Patients receiving insulin glulisine via CSII did not significantly differ from those receiving insulin aspart via CSII with regard to the incidence of catheter occlusions or the rate of or time between catheter changes.






Similar content being viewed by others
References
Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004 May; 27(5): 1047–53
American Diabetes Association. Standards of medical care in diabetes — 2008. Diabetes Care 2008 Jan; 31 Suppl. 1: S12–54
Roach P. New insulin analogues and routes of delivery: pharmacodynamic and clinical considerations. Clin Pharmacokinet 2008; 47(9): 595–610
Sanofi-aventis. Insulin glulisine (Apidra™) EU summary of product characteristics [online]. Available from URL: http://www.emea.europa.eu/humandocs/PDFs/EPAR/apidra/H-557-PI-en.pdf [Accessed 2009 Feb 12]
Sanofi-aventis. Insulin glulisine (Apidra™) US prescribing information [online]. Available from URL: http://products.sanofi-aventis.us/apidra/apidra.pdf [Accessed 2008 Nov 18]
Becker RH. Insulin glulisine complementing basal insulins: a review of structure and activity. Diabetes Technol Ther 2007 Feb; 9(1): 109–21
Hennige AM, Strack V, Metzinger E, et al. Effects of new insulin analogues HMR1964 (insulin glulisine) and HMR1423 on insulin receptors. Diabetologia 2005 Jul 29; 48: 1891–7
Hennige AM, Lehmann R, Weigert C, et al. Insulin glulisine: insulin receptor signaling characteristics in vivo. Diabetes 2005 Feb; 54(2): 361–6
Seipke G, Stammberger I. Effects of insulin glulisine on the insulin and IGF-1 receptor signalling cascades [abstract no. 1400-P]. Diabetes 2005; 54 Suppl. 1: A339–40
Rakatzi I, Ramrath S, Ledwig D, et al. A novel insulin analog with unique properties: Lys(B3), Glu(B29) insulin induces prominent activation of insulin receptor substrate 2, but marginal phosphorylation of insulin receptor substrate 1. Diabetes 2003 Sep; 52(9): 2227–38
Becker RH, Frick AD, Nosek L, et al. Dose-response relationship of insulin glulisine in subjects with type 1 diabetes. Diabetes Care 2007 Oct; 30(10): 2506–7
Becker RHA, Frick AD, Kapitza CK, et al. Pharmacodynamics (PD) and pharmacokinetics (PK) of insulin glulisine compared with insulin lispro (IL) and regular human insulin (RHI) in patients with type 2 diabetes [abstract no. 503-P]. Diabetes 2004 Jun; 53 Suppl. 2: A119. Plus poster presented at the 64th Annual Scientific Sessions of the American Diabetes Association; 2004 Jun 4–8; Orlando (FL)
Heise T, Nosek L, Spitzer H, et al. Insulin glulisine: a faster onset of action compared with insulin lispro. Diabetes Obes Metab 2007 Sep; 9(5): 746–53
Becker RH, Frick AD, Burger F, et al. Insulin glulisine, a new rapid-acting insulin analogue, displays a rapid time-action profile in obese non-diabetic subjects. Exp Clin Endocrinol Diabetes 2005 Sep; 113(8): 435–43
Luzio S, Peter R, Dunseath GJ, et al. A comparison of pre-prandial insulin glulisine versus insulin lispro in people with type 2 diabetes over a 12-h period. Diabetes Res Clin Pract 2008 Feb; 79(2): 269–75
Wernsing M, Atahi J, Larbig M, et al. Effect of insulin glulisine on postprandial plasma glucose levels in subjects with type 2 diabetes after a standard meal in comparison to insulin lispro [abstract no. 200-OR]. Diabetes 2008 Jun; 57 Suppl. 1: 60
Rave K, Klein O, Frick AD, et al. Advantage of premeal-injected insulin glulisine compared with regular human insulin in subjects with type 1 diabetes. Diabetes Care 2006 Aug; 29(8): 1812–7
Danne T, Becker RH, Heise T, et al. Pharmacokinetics, prandial glucose control, and safety of insulin glulisine in children and adolescents with type 1 diabetes. Diabetes Care 2005 Sep; 28(9): 2100–5
Garg SK, Rosenstock J, Ways K. Optimized basal-bolus insulin regimens in type 1 diabetes: insulin glulisine versus regular human insulin in combination with basal insulin glargine [published erratum appears in Endocr Pract 2005; 11 (2): 145]. Endocr Pract 2005; 11(1): 11–7
Kawamori R, Kadowaki T, Ishii H, et al. Efficacy and safety of insulin glulisine in Japanese patients with type 1 diabetes mellitus: using insulin glargine as basal insulin [abstract no. 2074-PO]. Diabetes 2008 Jun; 57 Suppl. 1: A573
Philotheou A, Arslanian S, Blatniczky L, et al. Efficacy and safety of insulin glulisine versus insulin lispro as part of a basal-bolus insulin regimen in children and adolescents with type 1 diabetes mellitus [abstract no. 950]. Diabetologia 2008 Sep; 51 Suppl. 1: 382
Fritsche A, Larbig M, Bohler S, et al. Superior efficacy of a basal-bolus regimen with insulins glargine and glulisine versus a two-injection pre-mixed insulin regimen in type 2 diabetes patients: results of the GINGER study [abstract no. 186]. Diabetologia 2008 Sep; 51 Suppl. 1: 83
Lankisch M, Ferlinz K, Leahy J, et al. Introducing a simplified approach to insulin therapy in type 2 diabetes: a comparison of two single-dose regimens of insulin glulisine plus insulin glargine and oral antidiabetic drugs. Diabetes Obes Metab 2008 Dec; 10(12): 1178–85
Rayman G, Profozic V, Middle M. Insulin glulisine imparts effective glycaemic control in patients with type 2 diabetes. Diabetes Res Clin Pract 2007 May; 76(2): 304–12
Dreyer M, Prager R, Robinson A, et al. Efficacy and safety of insulin glulisine in patients with type 1 diabetes. Horm Metab Res 2005 Nov; 37(11): 702–7
Dailey G, Rosenstock J, Moses RWK, et al. Insulin glulisine provides improved glycemic control in patients with type 2 diabetes. Diabetes Care 2004 Oct; 27(10): 2363–8
Woo JT, Kim SW, Baik SH, et al. Insulin glulisine improves glycaemic control in type 2 diabetic patients not optimally controlled with oral antidiabetic drugs: results of a randomised controlled study [abstract no. 991]. Diabetologia 2008 Sep; 51 Suppl. 1: 400
Hoogma RP, Schumicki D. Safety of insulin glulisine when given by continuous subcutaneous infusion using an external pump in patients with type 1 diabetes. Horm Metab Res 2006 Jun; 38(6): 429–33
Wynne AG, Nakhle S, Brusco OA, et al. Influence of preprandial vs postprandial insulin glulisine on A1C and body weight in patients with type 2 diabetes mellitus receiving basal insulin therapy: a multicenter, randomized, parallel, open-label study [abstract no. 2664-PO]. Diabetes 2008 Jun; 57 Suppl. 1: A722
Hohberg C, Forst T, Larbig M, et al. Effect of insulin glulisine on microvascular blood flow and endothelial function in the postprandial state. Diabetes Care 2008 May; 31(5): 1021–5
Horvath K, Bock G, Regittnig W, et al. Insulin glulisine, insulin lispro and regular human insulin show comparable end-organ metabolic effects: an exploratory study. Diabetes Obes Metab 2008 Jun; 10(6): 484–91
Burger F, Scholtz H, Frick A, et al. Pharmacodynamics and pharmacokinetics of insulin glulisine versus insulin lispro and regular human insulin in patients with type 1 diabetes [abstract no. 2350-PO]. Diabetes 2004 Jun; 53 Suppl. 2: A557
Becker RH, Frick AD. Clinical pharmacokinetics and pharmacodynamics of insulin glulisine. Clin Pharmacokinet 2008; 47(1): 7–20
Frick A, Becker RHA, Wessels D, et al. Pharmacokinetic and glucodynamic profiles of insulin glulisine following subcutaneous administration at various injection sites [abstract no. 511-P]. Diabetes 2003 Jun; 52 Suppl. 1: A119. Plus poster presented at the 63rd Annual Scientific Sessions of the American Diabetes Association; 2003 Jun 13–17; New Orleans (LA)
Frick AD, Scholtz H, Burger F, et al. Absorption of insulin glulisine when mixed with NPH insulin [abstract no. 1329-P]. Diabetes 2004 Jun; 53 Suppl. 2: A321. Plus poster presented at the 64th Annual Scientific Sessions of the American Diabetes Association; 2004 Jun 4–8; Orlando (FL)
Rave K, Nosek L, Heinemann L, et al. Insulin glulisine: pharmacokinetic and pharmacodynamic properties in comparison with insulin lispro and regular human insulin in Japanese and Caucasian volunteers [abstract no. 603-P]. Diabetes 2004 Jun 8; 53 (Suppl. 2): A143
Irie S, Becker R, Takahashi Y, et al. Comparison of pharmacodynamics and pharmacokinetics of single subcutaneous administration of insulin glulisine and regular human insulin in Japanese and Korean males using the euglycaemic clamp technique [abstract no. 2056-PO]. Diabetes 2008 Jun 1; 57 Suppl. 1: A568
Jaros M, Martinek V, Piechatzek R, et al. Pharmacokinetics of insulin glulisine in non-diabetic renally impaired patients [abstract no. 1330-P]. Diabetes 2004 Jun; 53 Suppl. 2: A321–2. Plus poster presented at the 64th Annual Scientific Sessions of the American Diabetes Association; 2004 Jun 4–8; Orlando (FL)
Sanofi-Aventis. Insulin glulisine administered pre-meal versus post-meal in adult subjects with type 2 diabetes mellitus receiving insulin glargine as basal insulin [ClinicalTrials. gov identifier NCT00135096]. US National Institutes of Health, ClinicalTrials.gov [online]. Available from URL: http://clinicaltrials.gov/ct2/show/NCT00135096 [Accessed 2008 Nov 13]
Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 2007 Sep; 30(9): 2181–6
Ulrich H, Snyder B, Garg SK. Combining insulins for optimal blood glucose control in type I and 2 diabetes: focus on insulin glulisine. Vasc Health Risk Manag 2007; 3(3): 245–54
International Diabetes Federation. Guideline for management of postmeal glucose [online]. Available from URL: http://www.idf.org/webdata/docs/Guideline_PMG_final.pdf [Accessed 2009 Feb 13]
Bell DS. Insulin therapy in diabetes mellitus: how can the currently available injectable insulins be most prudently and efficaciously utilised? Drugs 2007; 67(13): 1813–27
Chiasson JL, Josse RG, Gomis R, et al. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP-NIDDM trial. JAMA 2003 Jul 23; 290(4): 486–94
Novo Nordisk. Novolog: insulin aspart prescribing information [online]. Available from URL: http://www.novolog.com/Novolog_Prescribing_Info.pdf [Accessed 2009 Jan 6]
Eli Lilly and Company. Humalog: insulin lispro prescribing information [online]. Available from URL: http://pi.lilly.com/us/humalog-pen-pi.pdf [Accessed 2009 Jan 6]
US FDA. ICH topic E9: statistical principles for clinical trials [online]. Available from URL: http://www.fda.gov/cber/gdlns/ICHclinical.pdf [Accessed 2009 Jan 5]
Bergenstal RM, Johnson M, Powers MA, et al. Adjust to target in type 2 diabetes: comparison of a simple algorithm with carbohydrate counting for adjustment of mealtime insulin glulisine. Diabetes Care 2008 Jul; 31(7): 1305–10
Del Prato S, Nicolucci A, Vespasiani G. Optimising basal plus insulin therapy in type 2 diabetes by telecare assistance for self-monitoring of blood glucose: the ELEONOR study [abstract no. 1112]. Diabetologia 2008 Sep; 51 Suppl. 1: 452
Hermanns N, Kulzer B, Haak T. Dosing accuracy with a novel pen device (SoloSTAR) as performed by patients with diabetes in a clinical setting. Diabetes Technol Ther 2008 Aug; 10(4): 322–7
Singh C, Jovanovic L. Insulin analogues in the treatment of diabetes in pregnancy. Obstet Gynecol Clin North Am 2007 Jun; 34(2): 275–91, ix
Ryan PJ, Guesford PK, Hartshorn MF, et al. Maternal and fetal safety and outcomes in pregnancy complicated by insulin-requiring diabetes mellitus using basal and rapid-acting analog insulins [abstract no. 509-P]. Diabetes 2008 Jun; 57 Suppl. 1: A152
Lee F, Zhang Q, Mersey J, et al. Glycaemic control and costs with insulin glargine plus glulisine versus premix: a randomized, prospective, observational study [abstract no. 1003]. Diabetologia 2008 Sep; 51 Suppl. 1: A406
Walczak J, Dardzinski W, Kusy M, et al. The cost-effectiveness of insulin glulisine in type 2 diabetes in Poland [abstract no. PDB27]. Value Health 2008 May-2008 30; 11(3): A226
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: D.S.H. Bell, Division of Endocrinology and Metabolism, University of Alabama at Birmingham, Birmingham, Alabama, USA; B.W. Bode, Atlanta Diabetes Associates, Atlanta, Georgia, USA; K. Kaku, Department of Medicine, Kawasaki Medical School, Kurashiki, Japan; M. Landin-Olsson, Department of Endocrinology, University Hospital, Lund, Sweden; S.M. Setter, Department of Pharmacotherapy, Washington State University, Spokane, Washington, USA; G. Tamas, 1st Department of Medicine, Semmelweis University, Budapest, Hungary.
Data Selection
Sources: Medical literature published in any language since 1980 on ‘insulin glulisine’, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database of Wolters Kluwer Health ∣ Adis). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE, EMBASE or AdisBase search term was ‘insulin glulisine’. Searches were last updated 11 May 2009.
Selection: Studies in patients with type 1 or type 2 diabetes mellitus who received insulin glulisine. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Insulin glulisine, type 1 diabetes mellitus, type 2 diabetes mellitus, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability.
Rights and permissions
About this article
Cite this article
Garnock-Jones, K.P., Plosker, G.L. Insulin Glulisine. Drugs 69, 1035–1057 (2009). https://doi.org/10.2165/00003495-200969080-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200969080-00006