Abstract
-
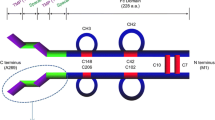
▴ Romiplostim is an Fc-peptide fusion protein (or ‘peptibody’) that stimulates megakaryopoiesis and thrombopoiesis by binding to, and activating, the thrombopoietin receptor.
-
▴ Because it has no sequence homology to thrombopoietin, romiplostim theoretically avoids the risk of eliciting cross-reacting, neutralizing antibodies to thrombopoietin.
-
▴ In well designed, 24-week, phase III trials, subcutaneous romiplostim was significantly more effective than placebo in achieving the primary endpoint of a protocol-defined durable platelet response in nonsplenectomized (61% vs 5%) or splenectomized (38% vs 0%) adults with chronic immune (idiopathic) thrombocytopenic purpura (ITP).
-
▴ Romiplostim was also significantly more effective than placebo with regard to a number of secondary endpoints, including the proportions of patients with an overall (durable plus transient) platelet response or who required ITP rescue medications. The majority of romiplostim-treated patients receiving concurrent ITP drugs were able to reduce or discontinue these therapies.
-
▴ Platelet response was maintained by most patients during longer-term treatment with romiplostim for up to 3 or 4 years in an open-label extension study.
-
▴ Romiplostim was generally well tolerated. Almost all adverse events in the phase III studies were of mild-to-moderate intensity; most were unrelated to treatment. Longer-term treatment with romiplostim had an adverse event profile consistent with that observed in the phase III studies.


Similar content being viewed by others
References
Fogarty PF, Segal JB. The epidemiology of immune thrombo-cytopenic purpura. Curr Opin Hematol 2007 Sep; 14(5): 515–9
Bussel J. Treatment of immune thrombocytopenic purpura in adults. Semin Hematol 2006; 43 Suppl. 5: S3–10
Psaila B, Bussel JB. Immune thrombocytopenic purpura. Hematol Oncol Clin North Am 2007; 21(4): 743–59
Cines DB, McMillan R. Pathogenesis of chronic immune thrombocytopenic purpura. Curr Opin Hematol 2007; 14: 511–4
Godeau B, Provan D, Bussel J. Immune thrombocytopenic purpura in adults. Curr Opin Hematol 2007 Sep; 14(5): 535–56
Kuter DJ, Bussel JB, Lyons RM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet 2008 Feb 2; 371(9610): 395–403
Newland A, Caulier MT, Kappers-Klunne M, et al. An open-label, unit dose-finding study of AMG 531, a novel thrombopoiesis-stimulating peptibody, in patients with immune thrombocytopenic purpura. Br J Haematol 2006 Nov; 135(4): 547–53
Panzer S. New therapeutic options for adult chronic immune thrombocytopenic purpura: a brief review. Vox Sang 2008 Jan; 94(1): 1–5
Rice L. Drug evaluation: AMG-531 for the treatment of thrombocytopenias. Curr Opin Investig Drugs 2006 Sep; 7(9): 834–41
Kaushansky K. The molecular mechanisms that control thrombopoiesis. J Clin Invest 2005 Dec; 115(12): 3339–47
Evangelista ML, Amadori S, Stasi R. Biologic aspects of thrombopoietin and the development of novel thrombopoietic agents for clinical use. Curr Drug Discov Technol 2007 Oct; 4(3): 162–73
Kaushansky K. Lineage-specific hematopoietic growth factors. N Engl J Med 2006 May 11; 354(19): 2034–45
Kuter DJ. New thrombopoietic growth factors. Blood 2007 Jun 1; 109(11): 4607–16
Andemariam B, Bussel J. New therapies for immune thrombocytopenic purpura. Curr Opin Hematol 2007; 14: 427–31
Wang B, Nichol JL, Sullivan JT. Pharmacodynamics and pharmacokinetics of AMG 531, a novel thrombopoietin receptor ligand. Clin Pharmacol Ther 2004 Dec; 76(6): 628–38
Broudy VC, Lin NL. AMG531 stimulates megakaryopoiesis in vitro by binding to Mpl. Cytokine 2004 Jan 21; 25(2): 52–60
Nichol JL. AMG 531: an investigational thrombopoiesis-stimulating peptibody. Pediatr Blood Cancer 2006 Oct 15; 47 (5 Suppl.): 723–5
Kumagai Y, Fujita T, Ozaki M, et al. Pharmacodynamics and pharmacokinetics of AMG 531, a thrombopoiesis-stimulating peptibody, in healthy Japanese subjects: a randomized, placebo-controlled study. J Clin Pharmacol 2007 Dec; 47(12): 1489–97
Bussel JB, Kuter DJ, George JN, et al. AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. N Engl J Med 2006 Oct 19; 355(16): 1672–81
Nplate™ (romiplostim) for subcutaneous injection. Prescribing information. Amgen Inc., Thousand Oaks (CA) [online]. Available from URL: http://www.nplate.com[Accessed 2008 Nov 28]
Bussel JB, Kuter DJ, Pullarkat V, et al. Safety and efficacy of long-term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood 2008 Nov 3
Pullarkat V, Gernsheimer TB, De Wolf JThM, et al. Reduction in immunoglobulin (IVIG or anti D) use in patients with chronic immune thrombocytopenic purpura receiving AMG 531: results from two phase 3 randomized placebo-controlled trials. Blood (ASH Annual Meeting Abstracts) 2007; 110: abstract 1304. Plus poster presented at the 49th Annual Meeting and Exposition of the American Society of Hematology; 2007 Dec 8–11; Atlanta (GA)
US Food and Drug Administration. FDA Advisory Committee briefing document: Oncologic Drugs Advisory Committee March 12, 2008. Biological License Application (BLA) 125268: romiplostim for the treatment of thrombocytopenia in certain adult patients with chronic immune (idiopathic) thrombocytopenia purpura. [online]. Available from URL: http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4345b1-01-FDA.pdf[Accessed 2008 Mar 27]
Kuter DJ, Bussel JB, Newland A, et al. Long-term treatment with romiplostim in patients with chronic immune thrombocytopenic purpura (ITP): 3-year update from an open-label extension study. Blood (ASH Annual Meeting Abstracts) 2008; 112: abstract 402. Oral presentation at the 50th Annual Meeting and Exposition of the American Society of Hematology; 2008 Dec 6–9; San Francisco (CA)
Data on file, Amgen (Europe) GmbH, 2009
George JN, Mathias SD, Go RS, et al. Improved quality of life for romiplostim-treated patients with chronic immune thrombocytopenic purpura: results from two randomized, placebo-controlled trials. Br J Haematol 2009 Feb; 144(3): 409–15
Lyons R, George JN, Lefrere F, et al. Evaluation of AMG 531 safety in splenectomized (S) and nonsplenectomized (NS) patients with chronic immune thrombocytopenic purpura (ITP) in two randomized placebo-controlled phase 3 studies. Blood (ASH Annual Meeting Abstracts) 2007; 110: abstract 1300. Plus poster presented at the 49th Annual Meeting and Exposition of the American Society of Hematology; 2007 Dec 8–11; Atlanta (GA)
Tarantino M, Sunkara U, George J, et al. Evaluation of bleeding and thrombotic events during long-term use of romiplostim in patients with chronic immune thrombocytopenic purpura. Blood (ASH Annual Meeting Abstracts) 2008; 112: abstract 3422. Plus poster presented at the 50th Annual Meeting and Exposition of the American Society of Hematology; 2008 Dec 6–9; San Francisco (CA)
Center for Drug Evaluation and Research. Application number: BLA 125268: summary review [online]. Available from URL: http://www.fda.gov/cder/foi/nda/2008/125268s000_sumR.pdf[Accessed 2008 Dec 1]
Jawa V, Hokom M, Hu J, et al. Low immunogenicity to romiplostim in clinical studies with ITP subjects. Blood (ASH Annual Meeting Abstracts) 2008; 112: abstract 3425. Plus poster presented at the 50th Annual Meeting and Exposition of the American Society of Hematology; 2008 Dec 6–9; San Francisco (CA)
Liebman H, Henry D, Lefrere F, et al. Long-term safety profile of romiplostim in patients with chronic immune thrombocytopenic purpura (ITP). Blood (ASH Annual Meeting Abstracts) 2008; 112: abstract 3415. Plus poster presented at the 50th Annual Meeting and Exposition of the American Society of Hematology; 2008 Dec 6–9; San Francisco (CA)
Amgen. Nplate® approved in the European Union for the treatment of chronic immune (idiopathic) thrombocytopenic purpura (ITP) [media release]. 2009 Feb 6 [online]. Available from URL: http://www.amgen.com/media/media_pr_detail.jsp?year=2009&=1253720
Acknowledgments and Disclosures
The manuscript was reviewed by: B. Godeau, Médecine Interne, Hôpital Henri-Mondor, Assistance Publique-Hôpitaux de Paris, Université Paris, Créteil, Paris, France; R. Stasi, Department of Medical Sciences, Ospedale “Regina Apostolorum”, Albano Laziale, Rome, Italy.
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturer of the agent under review was offered an opportunity to comment on this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Frampton, J.E., Lyseng-Williamson, K.A. Romiplostim. Drugs 69, 307–317 (2009). https://doi.org/10.2165/00003495-200969030-00006
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200969030-00006